Medical device and MedTech insights, news, tips and more
AVITA Medical’s “Spray-On Skin” Approved to Treat Serious Burns
September 25, 2018
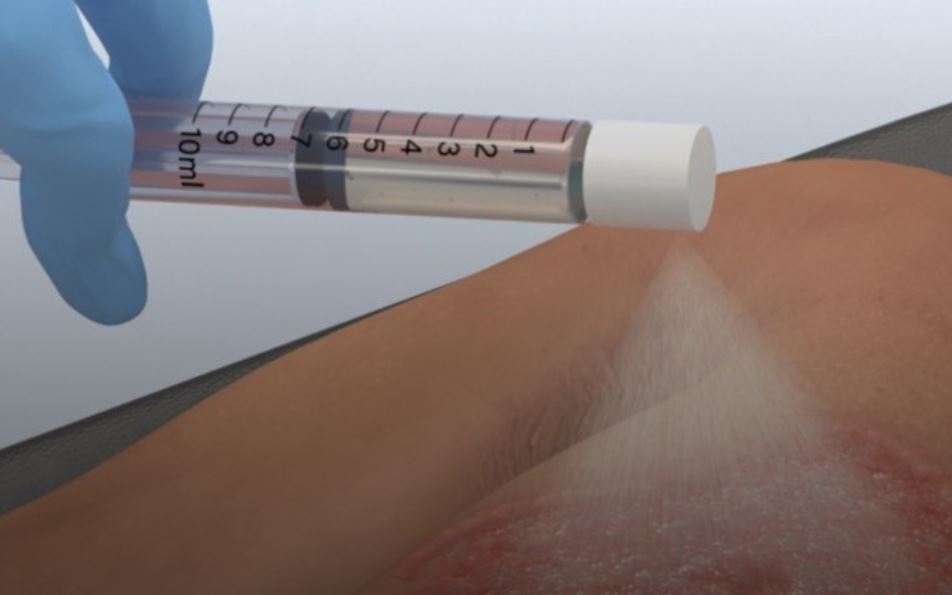
 AVITA Medical, a Valencia, California firm, won FDA approval for its remarkable RECELL Autologous Cell Harvesting Device for serious burns in adult patients. In preparation for treatment, a small healthy piece of the patient’s skin sample is taken, from which the so-called “Spray-On Skin” preparation is made. The process only takes 30 minutes, so it can be used soon after a burn occurs and unlike traditional autografts doesn’t require a lot of skin to be harvested (only 1/80 the size of the wound), though it can be used alongside autografts. Not only is this easier on patients, it is easier and less costly on the providers.
AVITA Medical, a Valencia, California firm, won FDA approval for its remarkable RECELL Autologous Cell Harvesting Device for serious burns in adult patients. In preparation for treatment, a small healthy piece of the patient’s skin sample is taken, from which the so-called “Spray-On Skin” preparation is made. The process only takes 30 minutes, so it can be used soon after a burn occurs and unlike traditional autografts doesn’t require a lot of skin to be harvested (only 1/80 the size of the wound), though it can be used alongside autografts. Not only is this easier on patients, it is easier and less costly on the providers.
The process of creating the therapeutic spray involves separating the sampled skin and treating it so that the outcome contains keratinocytes, fibroblasts, and melanocytes, cells that that help wounds heal. Since these are sprayed evenly across burn wounds, they are able to penetrate and settle throughout without requiring terribly precise application.
“Today’s approval of the RECELL System is a significant advancement in how we treat patients with burns,” in a published statement said James H Holmes IV, MD, FACS, Wake Forest Baptist Medical Center, Winston-Salem, North Carolina. “Dramatically reducing the amount of donor skin needed to treat second- and third-degree burns has important implications for pain, scarring and costs of care, while still providing comparable healing to the current standard of care. Additionally, the potential reduction in mortality is extremely promising.”
Read More at the Source: “Spray-On Skin” from AVITA Medical Approved to Treat Serious Burns | Medgadget
By: Medgadget
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
