Medical device and MedTech insights, news, tips and more
BioCardia Announces FDA Clearance for Morph DNA Catheter to Guide Cell Delivery into Heart Tissue
January 20, 2020
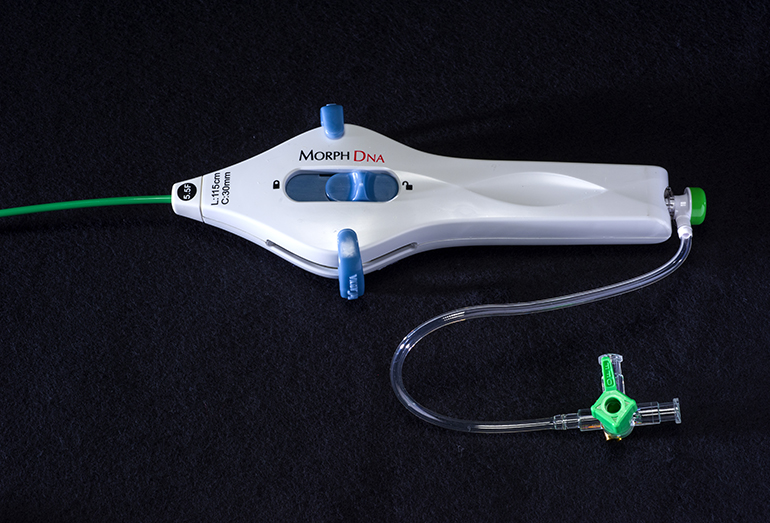
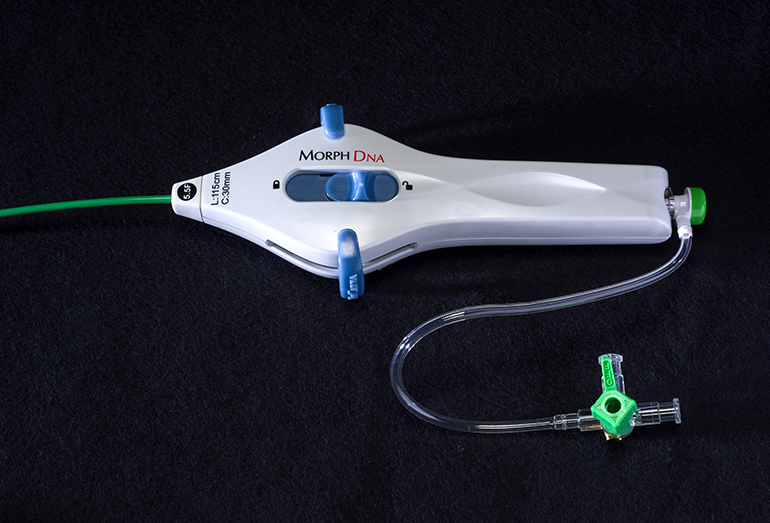
BioCardia (BCDA)®, Inc. [OTC: BCDA], a leader in the development of comprehensive solutions for cardiovascular regenerative therapies, today announced the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for the Morph® DNA deflectable guide catheter used to guide the Helix™ Biotherapeutic Delivery System during CardiAMP™ cell therapy delivery in the heart.
The Morph DNA deflectable guide catheter is designed to enhance procedural control of therapeutic catheters, such as the Helix system, during delivery of cell therapy. It is intended to provide superior ease of use through bidirectional deflection, reduced torque build up or whip, enhanced fluoroscopic visibility, and improved ergonomics. Helically-arranged pull-wires in the device, resembling the double helix of a DNA strand, increase catheter stability, rendering it virtually whipless around curves and within the heart. The device also features a new handle design with an adjustable brake for finer catheter control during procedures.
“The Morph DNA deflectable guide enables navigation within the cardiac chambers without building up torque. This is hugely beneficial for controlling the delivery of therapeutic catheters, such as Helix. The ergonomic features simplify the handling and can reduce procedural delays,” said Gerald Koenig, MD, PhD, cardiologist and principal investigator of the CardiAMP Heart Failure Trial at Henry Ford Health System.
“Our investigational Helix delivery system using the original Morph technology has shown a great safety profile and has been shown to be dramatically more efficient in helping the heart retain cell therapy when compared to other leading delivery approaches,” said BioCardia CEO Peter Altman, PhD. “The new Morph DNA device is an elegant product intended to further enhance the performance of our biotherapeutic delivery capabilities, and through these, our CardiAMP and CardiALLO cell therapy programs.”
About BioCardia
BioCardia, Inc., headquartered in San Carlos, California, is developing regenerative biologic therapies to treat cardiovascular disease. CardiAMP™ and CardiALLO™ cell therapies are the Company’s biotherapeutic product candidates in clinical development. The Company’s current products include the Helix™ Biotherapeutic Delivery System and the Morph® steerable guide and sheath catheter portfolio, including the new AVANCE™ Steerable Introducer family. BioCardia also partners with other biotherapeutic companies to provide its Helix systems and clinical support to their programs studying therapies for the treatment of heart failure, chronic myocardial ischemia and acute myocardial infarction.
See Full Press Release: Biocardia Announces FDA Clearance for Morph DNA Deflectable Guide Catheter | Seeking Alpha
Written by: BioCardia

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.