Biologics, Wound Care, Infection Prevention
Stimlabs® LLC Announces Launch of Relese® – A Uniquely Fenestrated Allograft for Chronic and Acute Wounds
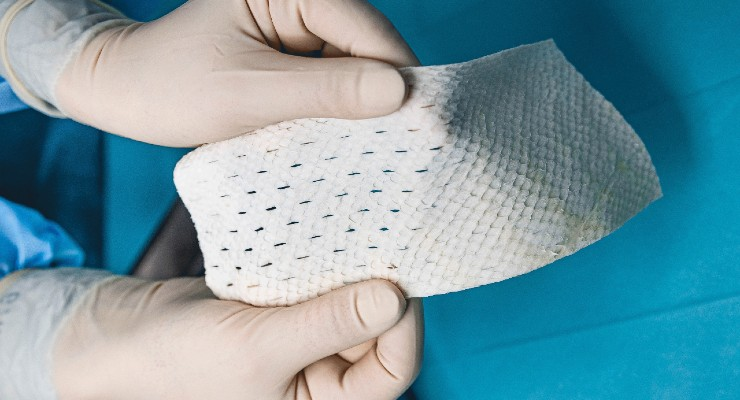
Stimlabs LLC, (“StimLabs”), a leader in regenerative medicine, announced the launch of Relese, a fenestrated dehydrated complete human placental membrane (dCHPM) allograft for chronic and acute wounds. As the latest solution in StimLabs’ growing suite of dCHPM allografts, Relese provides a selective barrier with channels that allow wound fluid to drain while also protecting the wound…
Read MoreKerecis to Donate FDA-Approved Fish Skin for Burn Victims of Maui Wildfires
Kerecis®, the company pioneering the use of fish skin and fatty acids for tissue regeneration and protection, is donating its GraftGuide® fish-skin burn product for victims of the fires on Maui, Hawaii. Qualified medical personnel wanting to get fish-skin burn-treatment products for their patients should call 703-287-8752 or email wildfires@kerecis.com. Kerecis is dispatching a specialist to train…
Read MoreAllosource Receives FDA 510(K) Clearance for Aceconnex™ Pre-Sutured Fascia for Hip Labral Reconstruction and Augmentation
AlloSource®, one of the largest allograft providers creating innovative cellular and tissue products to help surgeons heal their patients, today announced the U.S. Food and Drug Administration’s 510(K) clearance of AceConnex Pre-Sutured Fascia for hip labral reconstruction and augmentation. This product reinforces AlloSource’s commitment to providing innovative products to support the overall sports medicine market,…
Read MoreColoplast Announces Agreement to Acquire Kerecis and Raises Long-term Growth Expectations
Coloplast has signed an agreement to acquire Kerecis, an innovative, fast-growing company in the biologics wound care segment, for up to USD 1.3 billion (around DKK 8.9 billion), of which USD 1.2 billion (around DKK 8.2 billion) is an upfront cash payment See Full Press Release at the Source: Coloplast announces agreement to acquire Kerecis and…
Read MoreCresilon Receives First FDA Clearance For Human Use of Hemostatic Gel Technology
Cresilon, Inc. (“Cresilon”), a Brooklyn-based biotechnology company focused on hemostatic medical device technologies, today announced that it has been granted 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for Cresilon Hemostatic Gel™ (“CHG™”). This marks Cresilon’s first FDA clearance for human use, validating its revolutionary hemostatic gel technology and the company’s global mission to…
Read MoreCandela Medical Receives FDA Clearance for Vbeam 595 nm Pulsed Dye Laser, a Treatment for Port Wine Stains and Hemangiomas in Pediatric Cases
Candela Corporation (Candela), a leading global medical aesthetic device company headquartered in Marlborough, MA, today announced that the Vbeam Family of Pulsed Dye Lasers (PDL) has expanded its FDA cleared indications for use of the 595 nm wavelength to include the pediatric population (from birth – 21 years of age) for treatment of cutaneous capillary malformations, also…
Read MoreCartessa Aesthetics Introduces Helix by DEKA, a Powerful Fusion of Skin Resurfacing Technology
In the growing category of non-invasive, energy-based aesthetic procedures, laser skin resurfacing occupies one of the largest shares. Laser resurfacing is an effective way to reduce the appearance of fine lines, wrinkles, scars, unwanted pigment, and improve skin’s tone and texture. Cartessa Aesthetics and its manufacturing partner, DEKA, have been at the forefront of skin…
Read MoreKerecis Combines Fish-Skin Graft and Silicone Cover for Wound Treatment
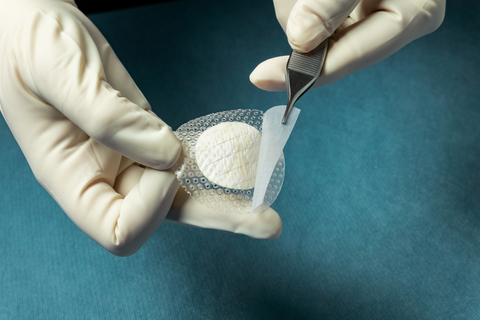
Kerecis® today announced MariGen® Shield, which integrates the company’s proven fish-skin graft with a silicone contact layer for treating chronic and complex wounds. The medical-fish-skin company also announced the results of a clinical study comparing the effectiveness of the Kerecis fish-skin grafts to a standard of care for diabetic foot ulcers. Both announcements were made at the Symposium…
Read MoreExtriCARE USA Introduces the extriCARE 3000 Pump to NPWT Market
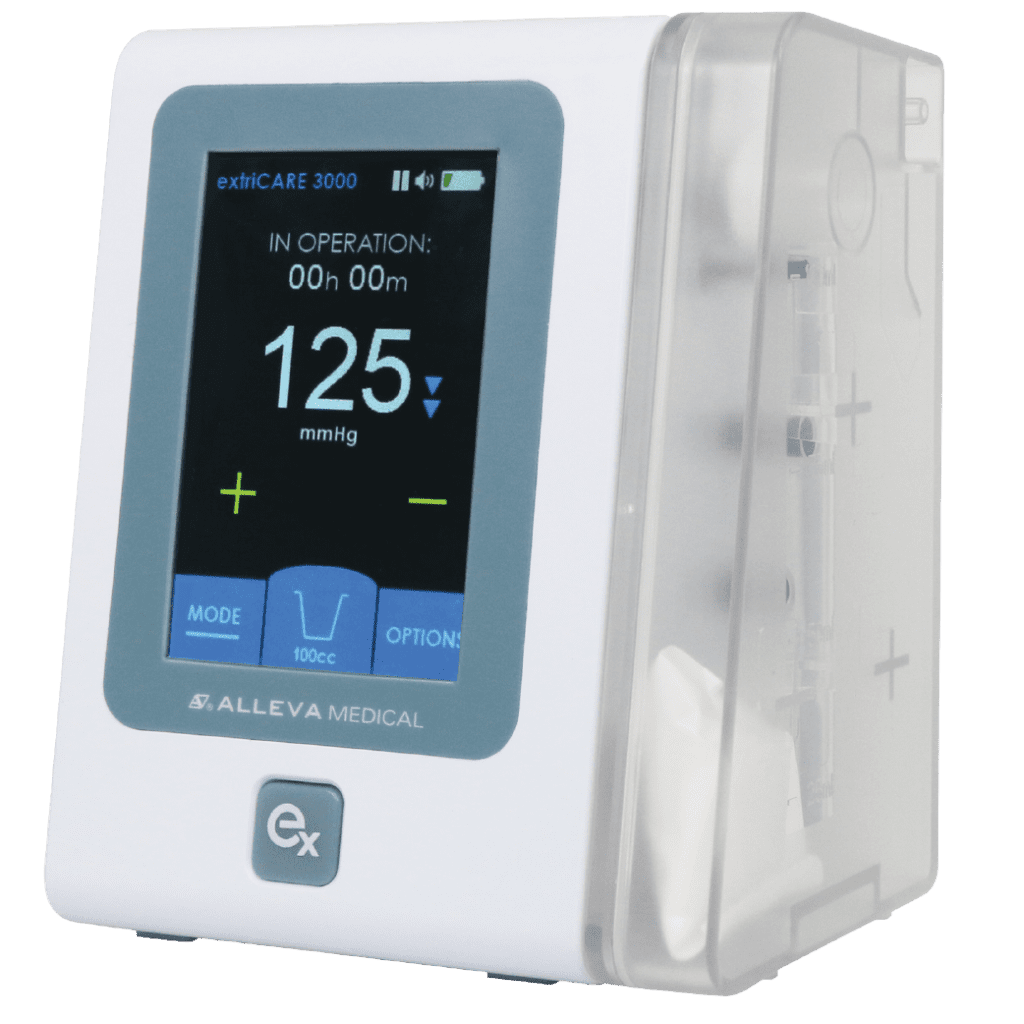
ExtriCARE USA, is pleased to announce that its innovative extriCARE 3000 pump has received FDA approval for use in the United States. The extriCARE 3000 pump is a state-of-the-art medical device designed to provide versatile and effective wound care treatment for patients. The extriCARE 3000 pump is a breakthrough in negative pressure wound therapy (NPWT)…
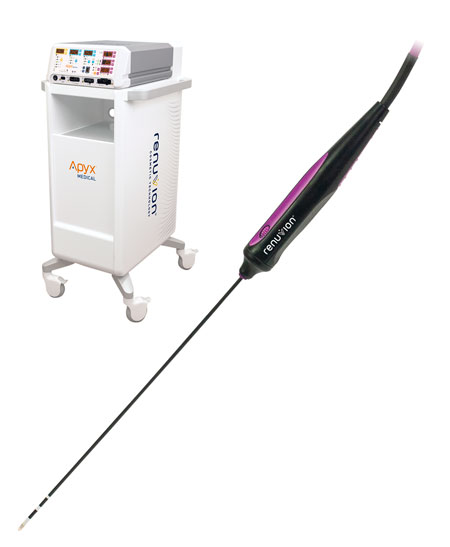
Read MoreApyx Medical Corporation Receives FDA 510(k) Clearance for the Use of Renuvion® to Coagulate and Contract Soft Tissues
Apyx Medical Corporation (NASDAQ:APYX), the manufacturer of a proprietary helium plasma and radiofrequency technology marketed and sold as Renuvion®, today announced it has received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for the use of the Renuvion APR Handpiece “for the delivery of radiofrequency energy and/or helium plasma where coagulation/contraction of soft…
Read More