Biologics, Wound Care, Infection Prevention
Advanced Sterilization Products Unveils the Fastest Biological Indicator for Hydrogen Peroxide Sterilization
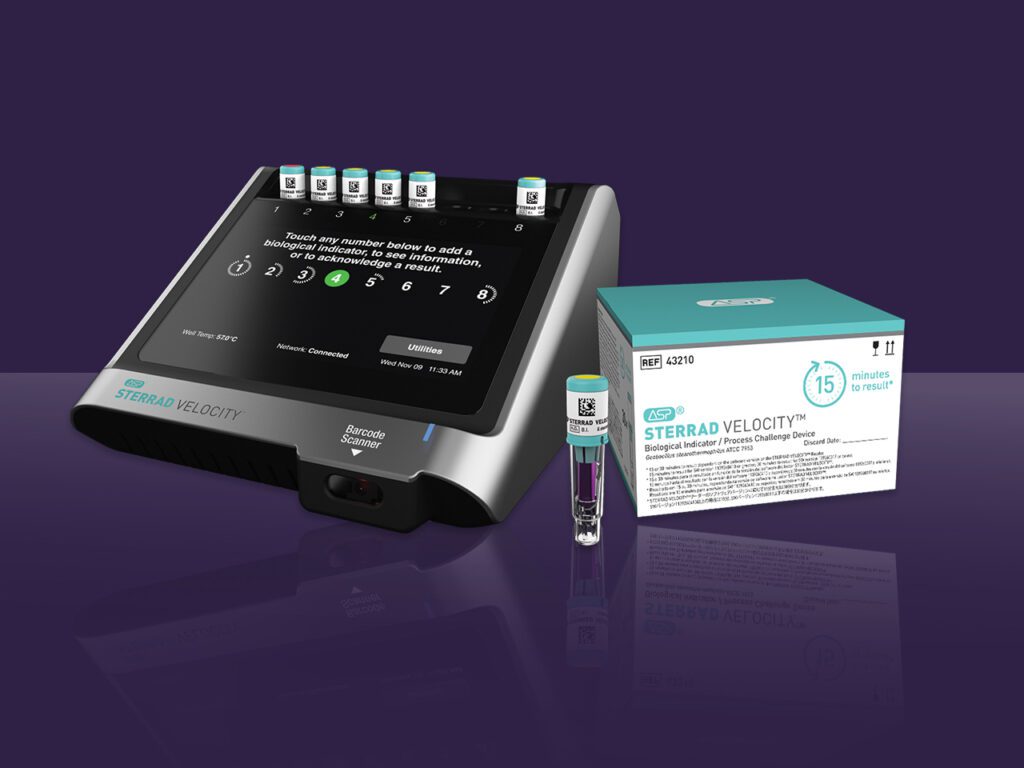
Advanced Sterilization Products (ASP) announced today it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for a 15-minute time to result on the STERRAD VELOCITY® Biological Indicator (BI)/Process Challenge Device (PCD) for use in STERRAD® Systems.* The 15-minute time to result is up to 38% faster than competition and offers the fastest way to…
Read MoreREVIAN Inc. Announces Issuance of Landmark Patent Covering the Use of Light to Stimulate the Body to Heal Itself
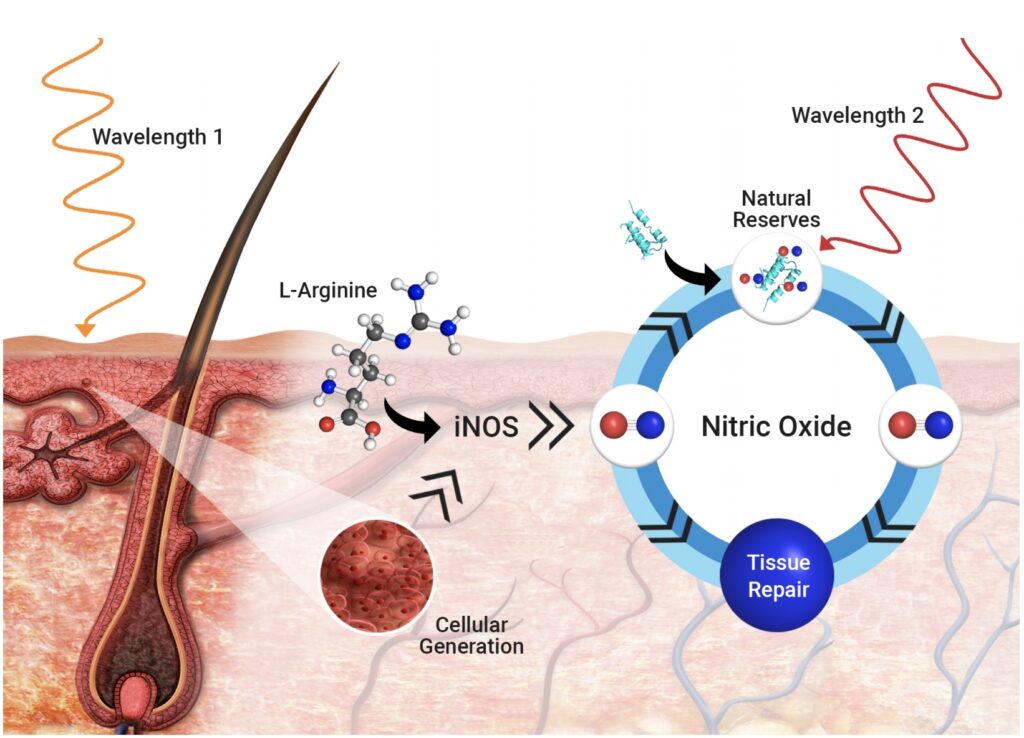
REVIAN Inc. (formerly PhotonMD Inc.), a medical technology company dedicated to stimulating the body’s natural processes to rejuvenate hair and skin with light, is pleased to announce that the United States Patent and Trademark Office (USPTO) issued U.S. Patent No. 10,265,258 – the seminal patent on the use of proprietary color combinations of light to…
Read MoreNanomesh Drug Delivery Provides Hope Against Global Antibiotic Resistance
The fight against global antibiotic resistance has taken a major step forward with scientists discovering a concept for fabricating nanomeshes as an effective drug delivery system for antibiotics. Health experts are increasingly concerned about the rise in medication resistant bacteria. Flinders University researchers and collaborators in Japan have produced a nanomesh that is capable of…
Read More3M Closes $6.7B Acelity Buyout – Acquisition Expands Presence in Advanced and Surgical Wound Care
3M announced it has completed the acquisition of Acelity, Inc. and its KCI subsidiaries worldwide from a consortium comprised of funds advised by Apax Partners (the Apax Funds), together with controlled affiliates of the Canada Pension Plan Investment Board (CPPIB) and the Public Sector Pension Investment Board (PSP Investments) for a total enterprise value of…
Read MoreSelf-Sterilizing Polymer Proves Effective Against Drug-Resistant Pathogens
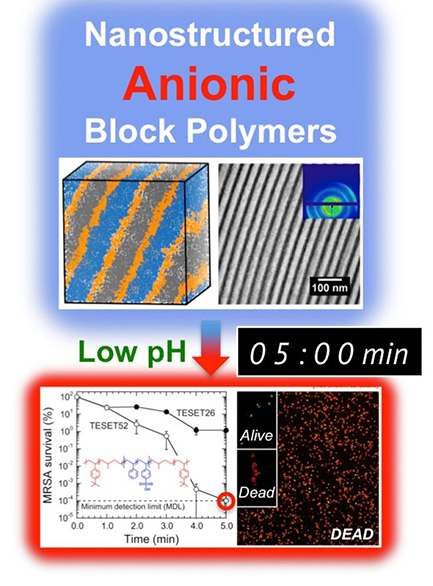
Researchers from North Carolina State University have found that an elastic polymer possesses broad-spectrum antimicrobial properties, allowing it to kill a range of viruses and drug-resistant bacteria in just minutes – including methicillin-resistant Staphylococcus aureus (MRSA). “We were exploring a different approach for creating antimicrobial materials when we observed some interesting behavior from this polymer and decided…
Read MoreCritical Innovations Receives Breakthrough Device Designation for FOAM Device
Critical Innovations announced earlier this week that the FDA has granted breakthrough device designation for its “Fast Onset Abdominal Management™ (F.O.A.M.™) device, which is designed to deliver a quickly-expanding foam to tamponade severe internal bleeding in trauma patients. F.O.A.M.™ research and development is supported by funding from U.S. Army Medical Materiel Development Activity (USAMMDA), through…
Read MoreSmith & Nephew to Buy Osiris Therapeutics for $660 Million
Smith & Nephew said today that it agreed to put $660 million on the table to acquire Osiris Therapeutics and its regenerative medicine portfolio. The British orthopedics and wound care giant said the $19-per-share deal is a 37% premium on the 90-day volume-weighted average for OSIR shares. It’s structured as a two-step tender offer, Smith & Nephew said,…
Read MoreFish-Skin Wound Care Company Gets Swiss Approval, Makes Acquisition
Kerecis, the company pioneering the use of fish skin in tissue regeneration, today received a notice from Swiss healthcare authorities that the company’s lead product, Kerecis Omega3 Wound, will be reimbursed in Switzerland. The company will also acquire the Swiss life-science company Phytoceuticals. The aim of the acquisition is to establish sales in Switzerland and strengthen Kerecis’ position…
Read MoreFDA Grants De Novo Authorization to Pressure Ulcer Risk Assessment Device
Bruin Biometrics, LLC has been granted U.S. Food and Drug Administration marketing authorization for the SEM Scanner, a wireless handheld device that is indicated for use as an adjunct to the standard of care when assessing patients who are at increased risk for pressure ulcers. The SEM Scanner is the world’s first FDA-authorized device to…
Read MoreAVITA Medical’s “Spray-On Skin” Approved to Treat Serious Burns
AVITA Medical, a Valencia, California firm, won FDA approval for its remarkable RECELL Autologous Cell Harvesting Device for serious burns in adult patients. In preparation for treatment, a small healthy piece of the patient’s skin sample is taken, from which the so-called “Spray-On Skin” preparation is made. The process only takes 30 minutes, so it…
Read More