Cardiovascular / Cardiology
Edwards Lifesciences gets approval for SAPIEN 3 TAVI valve in Europe
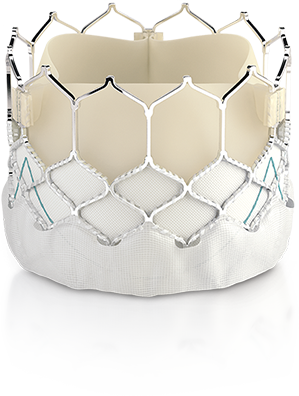
Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received CE Mark to expand use of the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients diagnosed with aortic stenosis who are at low risk for open-heart surgery.…
Read MoreSaranas Announces First Commercial Case With Early Bird® Bleed Monitoring System in the U.S.
Saranas, Inc. announced completion of the first U.S. commercial case using the Early Bird® Bleed Monitoring System for real-time detection and monitoring of endovascular bleed complications. Dr. Robert Kipperman, co-director of the Structural Heart Disease Program at Morristown Medical Center, and Dr. Bledi Zaku, cardiothoracic surgeon, successfully used the Early Bird to monitor for bleed complications…
Read MoreAnaconda Biomed Announces First-in-Human Study of Next-Generation Thrombectomy System
Anaconda Biomed, a medical technology company developing a next-generation thrombectomy system for the treatment of ischemic stroke, has announced the completion of initial patient cases in a first-in-human study at Hospital Vall d’Hebron in Barcelona. This 125-patient, prospective, multi-center study will assess system safety and reperfusion measured using the modified treatment in cerebral infarction (mTICI) score. Study…
Read MoreSiemens Healthineers Completes Corindus Vascular Robotics Acquisition for $1.1B
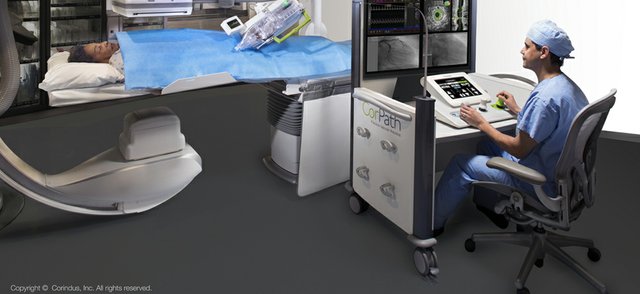
Siemens Healthineers AG completed the acquisition of 100 percent of Corindus Vascular Robotics, Inc. effective October 29, 2019. Prior to the closing of the acquisition, Corindus held a shareholders’ meeting on October 25, 2019, at which 87.5 percent of their stockholders approved the acquisition. The relevant governmental authorities previously granted the approvals required to complete…
Read More3D Printed Cells and Bioinks for Making Implantable Blood Vessels
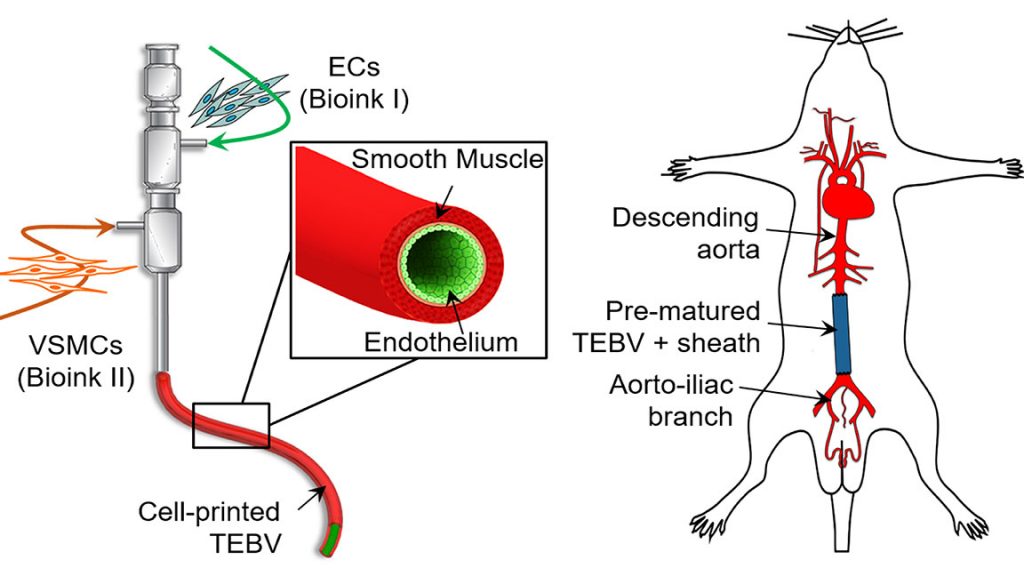
A biomimetic blood vessel was fabricated using a modified 3D cell printing technique and bioinks, which were formulated from smooth muscle cells from a human aorta and endothelial cells from an umbilical vein. The result is a fully functional blood vessel with a dual-layer architecture that outperforms existing engineered tissue and brings 3D-printed blood vessels…
Read MoreCorFlow Therapeutics Gains “Breakthrough Device Designation” for CoFI™ (COF-fee) System
CorFlow Therapeutics AG announced that the FDA has designated the company’s CoFI (CorFlow Controlled Flow Infusion) System as a “Breakthrough Device” with a broad indication-for-use statement: “The CoFI™System is indicated for diagnostic assessment of the coronary microcirculation immediately following PCI (“stenting”), and to be a platform for controlled infusion of therapeutic agents into the microcirculation with or without vessel occlusion.”…
Read MoreFDA Clears geko Muscle Pump Activator to Prevent VTE
Sky Medical Technology Ltd, has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its geko™ device for stimulation of the calf muscles to prevent venous thrombosis in non-surgical patients at risk for venous thromboembolism (VTE). This adds to Sky’s established 510k clearance for immediate post-surgical stimulation of calf muscles to prevent VTE, increasing blood circulation and…
Read MoreAngioDynamics Acquires Eximo Medical, Ltd. and its Innovative 355nm Laser Atherectomy Technology
AngioDynamics, Inc., a leading provider of innovative, minimally invasive medical devices for vascular access, peripheral vascular disease, and oncology, today announced that it has acquired Eximo Medical, Ltd., an early commercial stage, medical device company, and its proprietary 355nm wavelength laser-technology platform for $46 million in up-front consideration with up to $20 million of contingent…
Read MoreMedtronic Receives FDA Breakthrough Device Designation for Developing Stent Graft System to Treat Thoracoabdominal Aortic Aneurysm
Medtronic today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its Valiant® TAAA Stent Graft System for minimally invasive repair of thoracoabdominal aortic aneurysm (TAAA). A TAAA is a complex condition causing a bulging of the aorta, which extends from the chest down into to the abdomen.…
Read MoreCardiovascular Systems, Inc. Announces FDA Approval of the Coronary ViperWire Advance® With Flex Tip
Cardiovascular Systems, Inc. (CSI) (NASDAQ: CSII), a medical device company developing and commercializing innovative interventional treatment systems for patients with peripheral and coronary artery disease, today announced U.S. Food and Drug Administration (FDA) PMA approval of the new ViperWire Advance® Coronary Guide Wire with Flex Tip (ViperWire Advance with Flex Tip). ViperWire Advance with Flex Tip…
Read More