Cardiovascular / Cardiology
FDA Approves CVRx’s Neuromodulation Device for Heart Failure
CVRx, Inc., a private medical device company, announced today that it has received Premarket Approval (PMA) from the United States Food and Drug Administration (FDA) to market its BAROSTIM NEO device for heart failure in the United States. The FDA’s Center for Devices and Radiological Health (CDRH) approved the Company’s submission after a thorough review of…
Read MoreFDA Grants Breakthrough Device Designation to Ascyrus Medical Dissection Stent
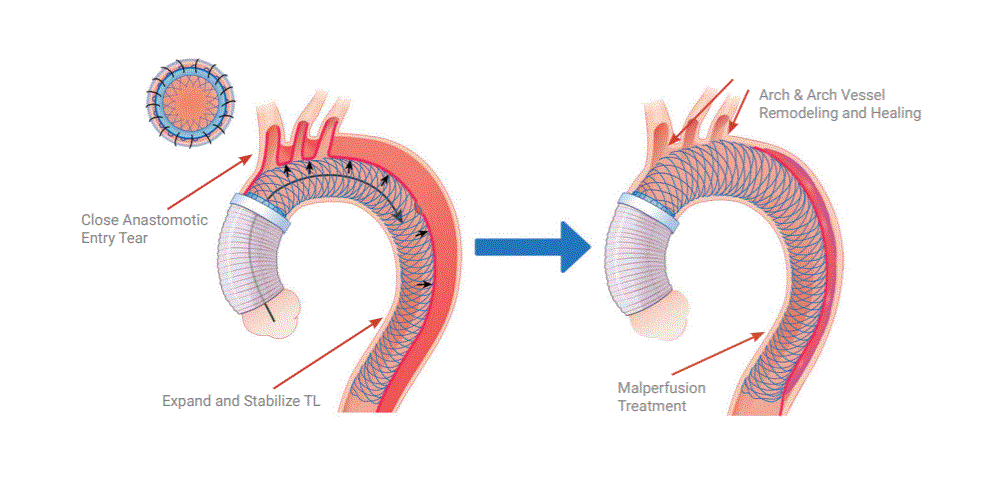
Ascyrus Medical announced yesterday that it has received Breakthrough Device Designation from the FDA for its Ascyrus Medical Dissection Stent (AMDS) to treat acute Type A aortic dissections. This designation serves as validation of the clinical importance of the AMDS and represents a significant milestone in the continued advancement of the technology globally. Ascyrus Medical…
Read MoreEndotronix Announces FDA Approval to Begin Landmark PROACTIVE-HF Pivotal Trial for Cordella PA Pressure Sensor System
Endotronix, Inc., a digital health and medical technology company dedicated to advancing the treatment of heart failure, today announced it has received conditional Investigational Device Exemption (IDE) approval from the U.S. Food and Drug Administration (FDA) to begin the multi-center PROACTIVE-HF trial of the Cordella™ Pulmonary Artery Pressure Sensor System (Cordella Sensor). The innovative trial…
Read MoreTransMedics Touts Long-Distance Organ Transport from Hawaii to North Carolina
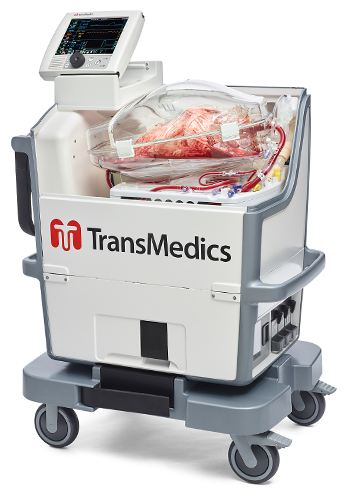
TransMedics Group, Inc., a medical technology company that is transforming organ transplant therapy for patients with end-stage lung, heart and liver failure, today announced the OCS Lung technology was used in two successful lung transplants using donor lungs from Hawaii that initially were declined for transplantation due to time and distance limitations of standard cold…
Read MoreMediCool Technologies Welcomes New CEO to Lead Development of Novel AFib Therapy
Electrophysiology Industry Veteran to Lead Early Stage Commercialization MediCool Technologies, an early stage medical device company developing a novel device to painlessly terminate cardiac arrhythmias, today announced that the Company has appointed Mr. Jeff Rynbrandt as President and Chief Executive Officer. Mr. Rynbrandt brings to the Company 25 year’s leadership across multiple disciplines in both…
Read MoreMaterialise Receives FDA Clearance for Cardiovascular Planning Software Suite
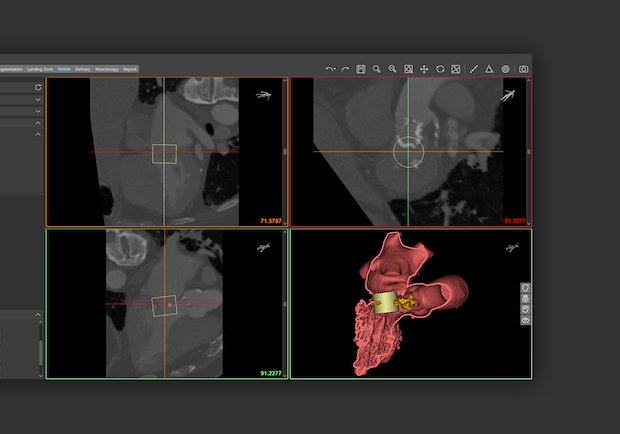
Materialise, a global 3D printing software and solutions company, has received FDA clearance for its Mimics Enlight cardiovascular planning software suite. The first release will support clinicians planning complex transcatheter mitral valve replacement (TMVR) procedures. Mimics Enlight is based on the strengths of Materialise’s Mimics Innovation Suite, which has helped clinicians produce patient-specific 3D models…
Read MoreRa Medical Launches Large Registry Trial for Peripheral Laser Device
Ra Medical said today that it launched a large registry study to follow patients treated with its laser device for restenosis in the peripheral arteries. Carldbad, Calif.-based Ra Medical’s Dabra device uses laser radiation ablation to bore through blockages in affected arteries. It’s designed to minimize damage to the surrounding vessel tissue. The 2,500-patient Results trial…
Read MoreCorMatrix Cor PATCH for Epicardial Tissue Repair Gets FDA Clearance
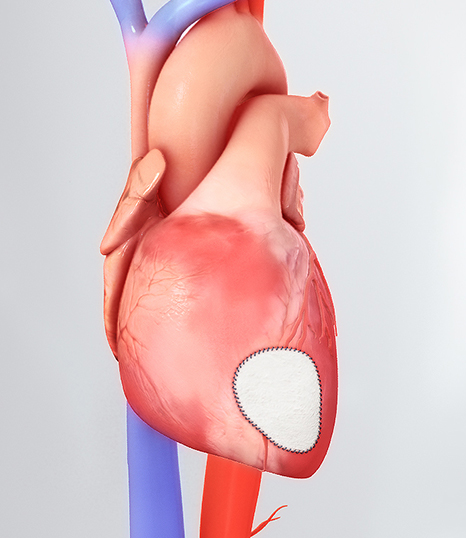
CorMatrix® Cardiovascular, Inc., a leading developer of regenerative cardiovascular medical devices, today announced FDA 510(k) clearance for the Cor™ PATCH. The Cor™ PATCH is indicated for epicardial tissue support and repair in adult patients. The Cor™ PATCH epicardial patch is the first epicardial cardiovascular medical device composed of first next generation CorMatrix® ECM® to be…
Read MoreZoll Buys AED Developer Cardiac Science
Cardiac Science Corporation, a leading provider of automated external defibrillators (AEDs), related services and accessories, today announced it has entered into a definitive agreement to be acquired by ZOLL® Medical Corporation, an Asahi Kasei Group Company that manufactures medical devices and related software solutions. CSC is a portfolio company of Aurora Resurgence, a Los Angeles-based…
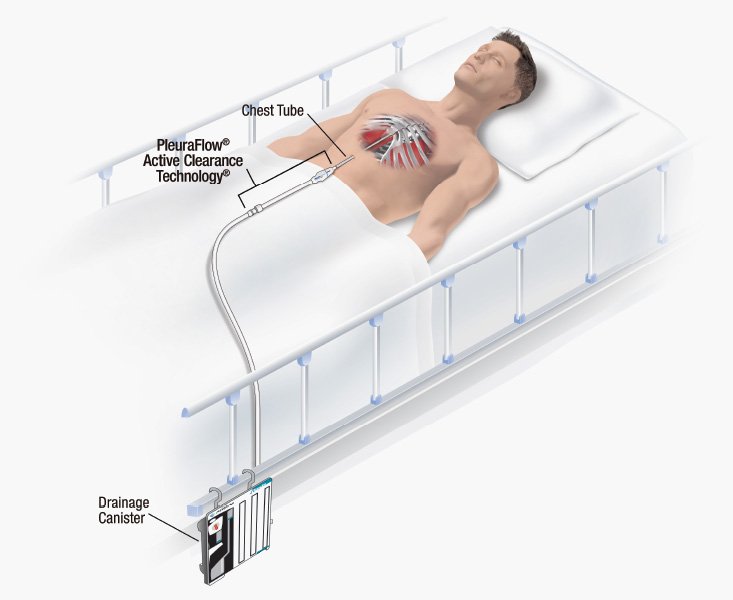
Read MoreClearFlow Announces Positive Trial Results for PleuraFlow Thoracic Evacuation Device
ClearFlow, Inc., a medical device company based in Anaheim, California, has announced that positive clinical trial results of its PleuraFlow® device were presented at the American Association for Thoracic Surgery (AATS) 99th Annual meeting on May 4 in Toronto, Canada. This new data stems from a trial evaluating the use of ClearFlow’s PleuraFlow® Active Clearance…
Read More