Cardiovascular / Cardiology
FDA Approves First New Therapy to Treat Heart Attacks in Years
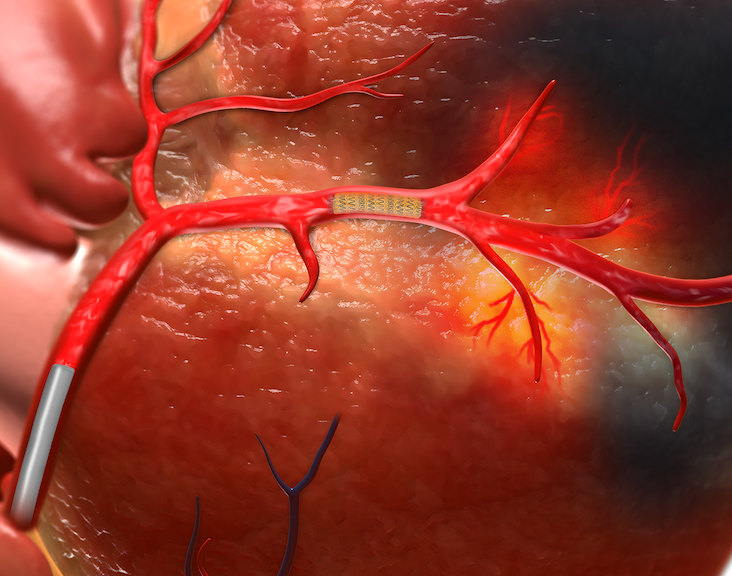
TherOx Inc. announced that the U.S. Food and Drug Administration (FDA) granted premarket approval for its SuperSaturated Oxygen (SSO2) Therapy. SSO2 Therapy provides interventional cardiologists with the first and only FDA-approved treatment beyond percutaneous coronary intervention (PCI) to significantly reduce muscle damage in heart attack patients. SSO2 Therapy delivers hyperbaric levels of oxygen directly to the ischemic…
Read MoreGenetesis CardioFlux Platform Receives FDA 510(k) Clearance
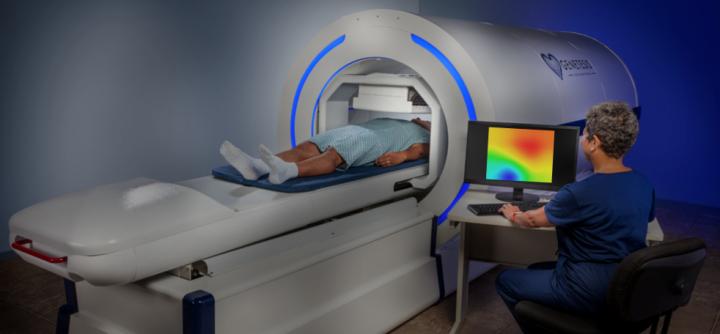
Genetesis, a company based in Mason, Ohio, won FDA clearance for its cardiac imaging offering that combines the CardioFlux magnetocardiograph with the the integrated Faraday Analytical Cloud (FAC). The technology is intended to be used by ER physicians and cardiologists to quickly assess patients presenting with chest pain, helping with triage and getting patients to receive…
Read MoreFDA Approves Breakthrough Optimizer Smart Device for Chronic Heart Failure
Impulse Dynamics, developer of the implantable Optimizer® Smart System for delivering CCM™ therapy, announced today that it has received approval from the United States Food and Drug Administration for its first-in-class Optimizer Smart System (link to FDA announcement: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm634103.htm). Designed to address a significant unmet medical need in heart failure, the Optimizer Smart System received Breakthrough…
Read MoreSonavex Receives FDA Clearance for Blood Flow Monitoring Technology
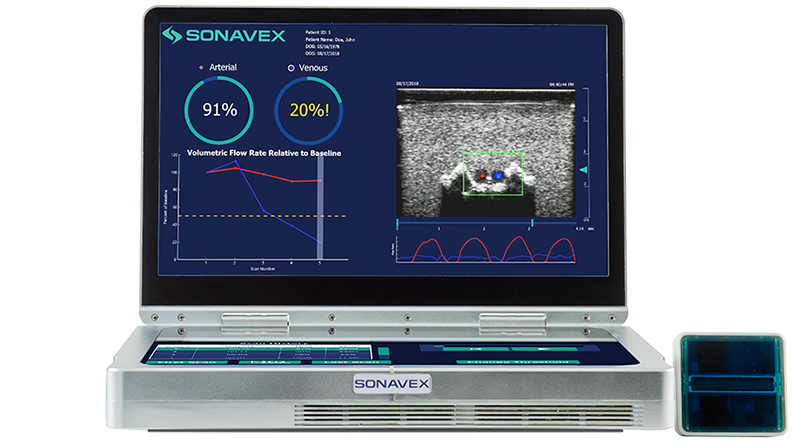
Following peripheral vascular and microvascular surgeries, it is important to be able to monitor how blood is flowing through the treated vessels and whether there may be an occlusion or compromise. This monitoring typically requires a nurse or a trained sonographer to use Doppler ultrasound, but now a new option is available. Sonavex, a company…
Read MoreSaranas Achieves De Novo FDA Clearance for Their Endovascular Monitoring System
Saranas Inc., a medical device company with innovative technology for real-time detection and monitoring of internal bleeding during endovascular procedures, today announced that it has been granted de novo classification by the U.S. Food and Drug Administration (FDA) for the Early Bird Bleed Monitoring System. According to a recent study of over 17,000 large-bore transcatheter…
Read MoreMassive Medtronic Pacemaker Recall is Class I Says FDA
The FDA has slapped the most serious designation on a recall of approximately 156,957 Medtronic dual-chamber pacemakers. The Fridley, Minn.-based company issued a recall in January on pacemakers with model names Adapta, Versa, Sensia, Relia, Attesta, Sphera, and Vitatron due to a software error that can result in a lack of pacing. Patients and physicians cannot predict whether and when this…
Read MoreItamar Medical Gets Favorable CMS Reimbursement Decision for WatchPAT Technology
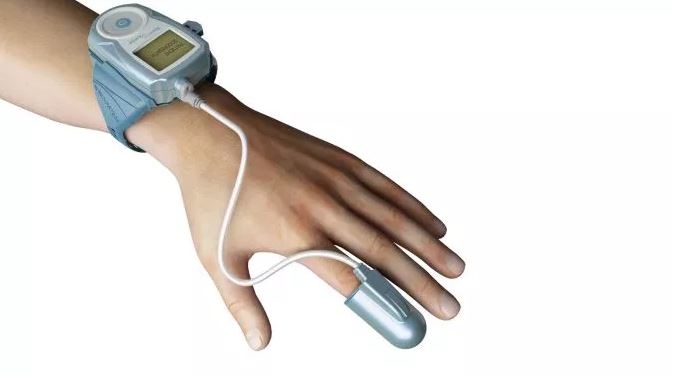
Itamar Medical Ltd. today announced the release of the 2019 Fee Schedule from the U.S. Centers for Medicare & Medicaid Services (CMS) that should support broad use of its WatchPAT technology. WatchPAT is the only product on the market today that uses Peripheral Arterial Tone technology, providing cardiologists and sleep physicians in the United States…
Read MoreCardiawave raises $8.2 million for non-invasive aortic stenosis device
Parisian startup Cardiawave has raised more than $8 million to boost development of its ultrasound device to treat calcific aortic stenosis. Nearly $3 million came from the European program Instrument PME, which finances disruptive European technologies developed by potentially high-growth companies. Family-controlled investment groups and the company’s traditional shareholders — Sofimac Innovation, Business Angels des Grandes Ecoles, Paris…
Read MorePeople who walk just 35 minutes a day may have less severe strokes
People who participate in light to moderate physical activity, such as walking at least four hours a week or swimming two to three hours a week, may have less severe strokes than people who are physically inactive, according to a study published in the September 19, 2018, online issue of Neurology, the medical journal of the…
Read MoreNew Apple Watch Will Have Built-In ECG, and It’s Already FDA Approved
At Apple’s annual September hardware launch event, Apple revealed a major medical advancement added to their newest update in the Apple Watch line: the Apple Watch Series 4. Among other updates including a larger screen and smaller bezel, the system boasts the first FDA approved direct to consumer ECG, or electrocardiogram, a medical device used…
Read More