Cardiovascular / Cardiology
FDA Issues Warning Letters to Medtronic CRM Plants in Puerto Rico, Minnesota
The US Food and Drug Administration (FDA) issued two warning letters to Medtronic on Tuesday related to manufacturing nonconformities that resulted in a defibrillator recall earlier this year. The recall of the Medtronic Cardiac Resynchronization Therapy with Defibrillation (CRT-Ds) and Implantable Cardiovert-Defibrillators (ICDs) models was initiated in January “due to a defect in the manufacturing…
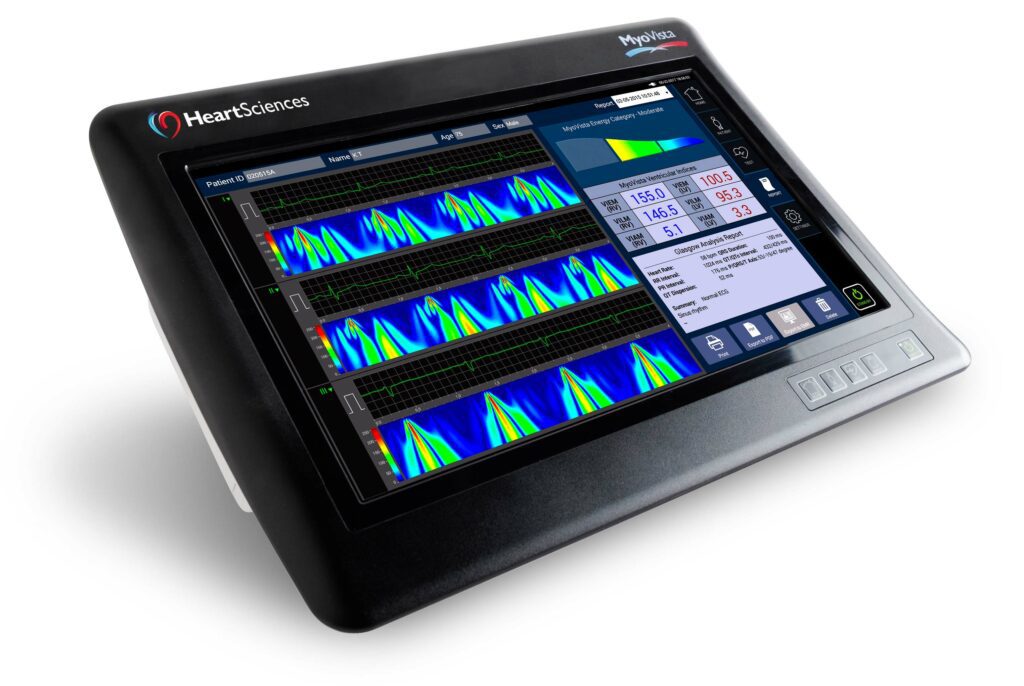
Read MoreRevolution in Electrocardiography Long in Coming, but MyoVista Fills the Bill
“Revolutionary!” and “Transformative!” are tossed around like so much jetsam in today’s spotlight-driven culture, and the medical field is not immune. But we believe a product that harvests multiple indicators of heart disease from a simple, inexpensive, first-line test has earned these superlatives. MyoVista wavECG by HeartSciences, which we saw introduced at this year’s ACC.18…
Read MoreVascular Changes after Smoking Hookah on par with Cigarettes
Smoking hookah for 30 minutes causes changes in arterial stiffness similar to what’s seen after someone smokes a cigarette, according to a study published in the American Journal of Cardiology. “Our findings challenge the concept that fruit-flavored hookah tobacco smoking is a healthier tobacco alternative. It is not,” lead author Mary Rezk-Hanna, PhD, an assistant professor at the…
Read MoreBioSig’s High-Fidelity Electrophysiology System Approved by FDA
BioSig Technologies, Inc. announced that the Company has received 510(k) clearance for its first product, PURE EP System, from the U.S. Food and Drug Administration (FDA). The non-invasive PURE EP System is a computerized system intended for acquiring, digitizing, amplifying, filtering, measuring and calculating, displaying, recording and storing of electrocardiographic and intracardiac signals for patients…
Read MoreBoston Scientific Spends $160M on Veniti’s Venous Stents
Boston Scientific is proving itself to have more stamina than the Energizer Bunny when it comes to making acquisitions. The Marlborough, MA-based company is now picking up Veniti, a firm that has developed a stent system for treating venous obstructive disease. The deal consists of $108 million up-front cash as well as up to $52 million in…
Read MoreWorld’s Smallest Clot Retriever for Strokes Receives CE Mark
Rapid Medical, based in Yokneam, Israel, won European regulatory approval to introduce its TIGERTRIEVER 13, the narrowest clot retriever now available for use in treating ischemic stroke. The device can be used in vessels as small as 1 millimeter in width and up to a maximum of 2.5 mm, where larger retrievers would be appropriate. It works…
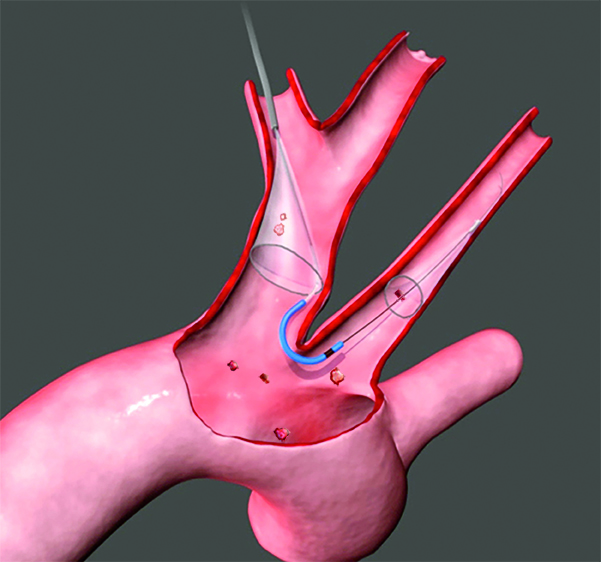
Read MoreBoston Scientific to Buy Claret Medical for up to $270 Million
Boston Scientific Corporation today announced it has signed an agreement to acquire Claret Medical, Inc., a privately-held company that has developed and commercialized the Sentinel® Cerebral Embolic Protection System. The device is used to protect the brain during certain interventional procedures, predominately in patients undergoing transcatheter aortic valve replacement (TAVR). The transaction price consists of $220 million in up-front…
Read MoreBoston Scientific to Pay $202 Million for Cryterion Medical
Boston Scientific Corporation today announced a definitive agreement to acquire Cryterion Medical, Inc., a privately-held company developing a single-shot cryoablation platform for the treatment of atrial fibrillation (AF). The addition of this cryoballoon platform positions the company as the first to have both cryothermal and radiofrequency (RF) single-shot, balloon-based ablation therapies in its portfolio. Boston…
Read MoreBIOLIFE4D Successfully 3D Bioprints Human Heart Tissue
BIOLIFE4D, a biotech pioneer leveraging advances in tissue engineering to 3D print human organs viable for transplant, today announced it has successfully demonstrated its ability to 3D bioprint human cardiac tissue – specifically, a human cardiac patch. The scientific milestone was accomplished within days of the May opening of the Company’s new research facility at JLABS in…
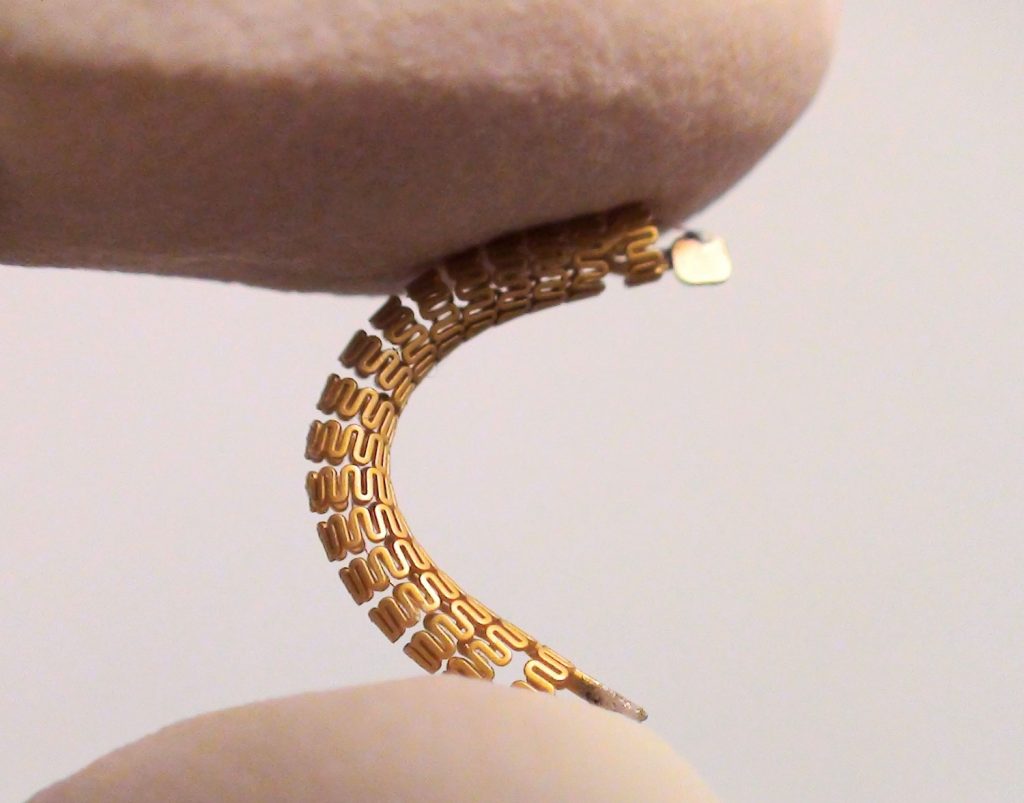
Read More“Smart Stent” Monitors Blood Flow, Detects Narrowing of Arteries
Vascular stents are some of the most commonly implanted medical devices, keeping millions of arteries open to uninterrupted blood flow. Though they tend to work well at first, too many end up being re-stenosed by new deposits of plaque and scar tissue often forms in the vicinity, blocking blood flow through the stent. Typically, renewed…
Read More