Cardiovascular / Cardiology
FDA Grants Clearance for UltraSight’s AI-Powered Cardiac Ultrasound Technology
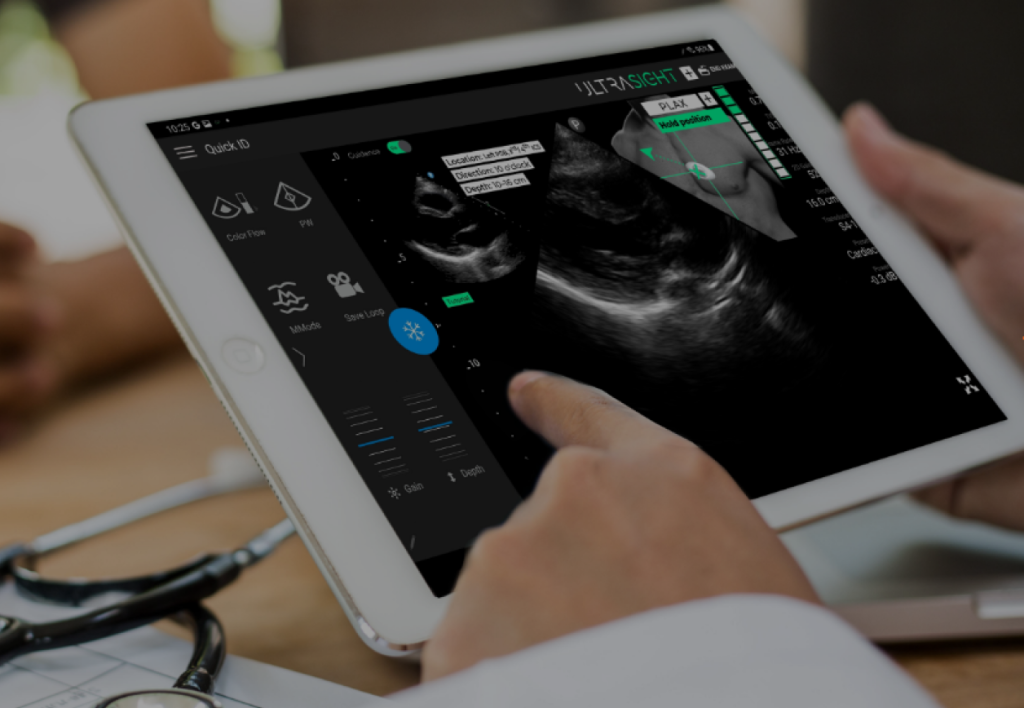
UltraSight, a digital health pioneer transforming cardiac imaging through the power of artificial intelligence, announced that it has been granted FDA clearance for its AI-powered ultrasound guidance technology. The UltraSight real-time AI guidance software can assist medical professionals without sonography experience in acquiring cardiac ultrasound images at the point of care in multiple settings, allowing for…
Read MoreFDA Clears Numares Health Cardiovascular Diagnostic Test and Core Technology Platform
The US Food and Drug Administration has cleared a Numares Health test, the AXINON® LDL-p Test System, as a new tool physicians can use to measure lipoproteins for patients at risk for cardiovascular disease. Currently, Numares is the only company in the US selling an FDA-cleared NMR test. The FDA clearance also includes the company’s…
Read MoreAccurKardia’s AccurECG™ Analysis System Receives FDA 510(k) Clearance
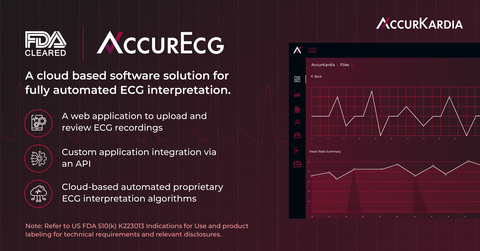
AccurKardia, a medical technology company delivering clinical-grade ECG interpretation software, announced today that its flagship product, the AccurECG™ Analysis System (“AccurECG” or the “System”), has been granted FDA 510(k) clearance. AccurECG™ is a cloud-based, device-agnostic and fully automated electrocardiogram (ECG) interpretation software platform. The groundbreaking AccurECG™ software provides an array of benefits, such as beat-by-beat analysis, ventricular/supraventricular…
Read MoreFDA Approves Innovative 4D Flow MRI Blood Flow Analysis Software from Cardio Flow Design Inc.
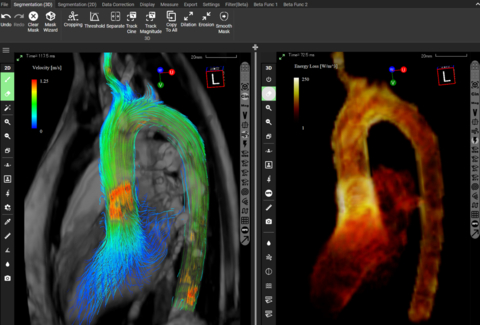
The US Food and Drug Administration has approved the use of iTFlow® in blood flow analysis. The FDA approval is significant as it recognizes the safety and effectiveness of iTFlow® in analyzing blood flow through 4D Flow MRI data, with the potential to enhance diagnostic accuracy for patients with cardiovascular diseases and heart conditions. The…
Read MoreInnova Vascular Earns FDA Clearance for Two New Thrombectomy Devices: Laguna Clot Retriever™ System and Malibu Aspiration Catheter™ System
Innova Vascular, Inc. announced today that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its Laguna Clot Retriever™ System and its Malibu Aspiration Catheter™ System for use in the peripheral vasculature. The two new devices are collectively known as the Laguna Thrombectomy System. “Removing clots quickly, safely, and in large volumes from the…
Read MorePaceMate Announces Collaboration with AliveCor to Offer Cutting-Edge Remote Monitoring Platform
PaceMate® announced today its collaboration with AliveCor®, the global leader in FDA-cleared personal electrocardiogram technology and remote patient management solutions for the cardiovascular industry. This collaboration will combine the strengths of PaceMate’s world-class cardiac remote monitoring platform, PaceMateLIVE™ – designed to improve clinical, operational, and financial outcomes – with AliveCor’s KardiaPro platform to capture and triage…
Read MoreCardiaCare Names Ken Nelson Chairman of its Board of Directors
CardiaCare, a clinical stage digital therapeutic company developing the world’s first non-invasive, neuromodulation wearable for atrial fibrillation treatment, announced today that Ken Nelson has been appointed Chairman of the Board. Mr. Nelson is a 20-year digital health, medical device, and remote patient monitoring executive with a stellar track record of building successful sales, marketing and commercialization teams…
Read MoreAngel Medical Systems, Creator of the Guardian™, First Implantable Cardiac Alert System, Changes Name to Avertix Medical Inc. and Welcomes New CEO and CFO to Drive Continued Growth and Success
Angel Medical Systems Inc. (dba AngelMed), a company focused on improving long-term management of high-risk coronary disease in patients who have survived one or more heart attacks, today announced it has changed its corporate name to Avertix Medical Inc. (Avertix), and appointed Tim Moran as President and Chief Executive Officer and Philip Tom as Executive Vice…
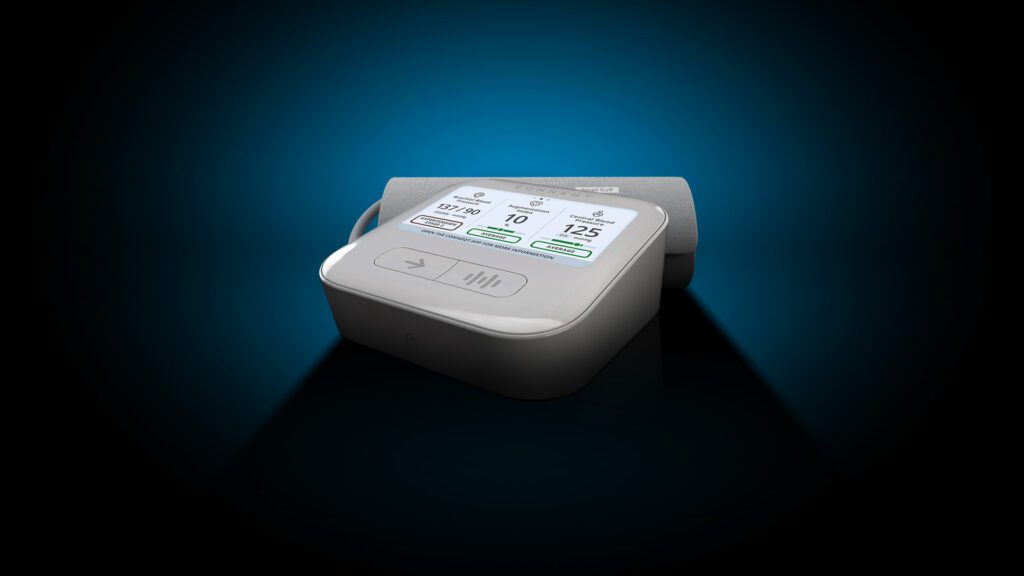
Read MoreCardieX Receives FDA 510(K) Clearance for a World-First Vascular Biometric Monitor – the CONNEQT Pulse
CardieX Limited (ASX: CDX) (CardieX, the Company), today announced its new arterial health monitor, the CONNEQT Pulse (Pulse), has received 510(k) clearance from the U.S. Food and Drug Administration (FDA). Pulse is the only vital signs monitor targeted at home, clinician, and clinical trial use that provides measurements of both brachial blood pressure (the pressure at…
Read MoreBlackSwan Vascular Wins FDA Premarket Approval of Innovative New Treatment for Peripheral Arterial Hemorrhages
Bay Area-based BlackSwan Vascular, Inc., a privately held company that is developing innovative therapies in endovascular embolization, is pleased to announce it has received FDA Premarket Approval (PMA) of its Lava® Liquid Embolic System (Lava® LES) for treatment of peripheral arterial hemorrhage. Lava LES, a nonadhesive injectable, is the first liquid embolic product approved by…
Read More