Diagnostics & Healthcare News
Opsens Inc. Announces 510(k) Clearance from the FDA to Market its Diastolic Pressure Algorithm
Opsens Inc. announces 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) to market its diastolic pressure algorithm (“dPR”). Coronary physiology has been in constant evolution with the expanded use of Fractional Flow Reserve (“FFR”) and the support of strong clinical data and cardiology societies recommendations. More recently, the option for coronary physiology without hyperemia…
Read MoreBaebies Announces CE Mark for FINDER, an Innovative Near-Patient Testing Platform for Glucose-6-Phosphate Dehydrogenase
Baebies is pleased to announce that FINDERTM, a near-patient testing platform, now has CE Mark as an In Vitro Diagnostic device (IVD) and is commercially available in Europe and other countries that recognize CE Mark. The CE-Marked platform includes an instrument and a cartridge, which tests for Glucose-6-Phosphate Dehydrogenase (G6PD) from low blood volume, a single drop…
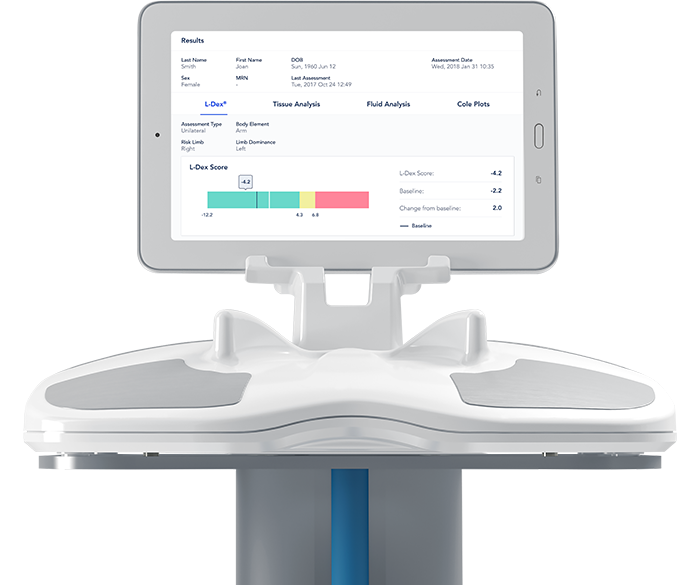
Read MoreImpediMed Receives FDA 510(k) Clearance of SOZO for Expanded Indication
ImpediMed Limited (ASX.IPD), a medical software technology company that non-invasively measures, monitors and manages fluid status and tissue composition using bioimpedance spectroscopy (BIS), recently announced the issuance of a further 510(k) clearance for SOZO® by the U.S. Food and Drug Administration (FDA). SOZO is the first device to gain FDA clearance for use as an…
Read MoreBeta Bionics Receives FDA Breakthrough Device Designation for the iLet Bionic Pancreas System
Beta Bionics, Inc. — a medical technology company developing and aiming to commercialize the world’s first fully automated bionic pancreas — today announced it has received Breakthrough Device designation from the U.S. Food and Drug Administration (FDA) for its investigational iLet Bionic Pancreas System. The iLet Bionic Pancreas System is a pocket-sized, wearable investigational medical…
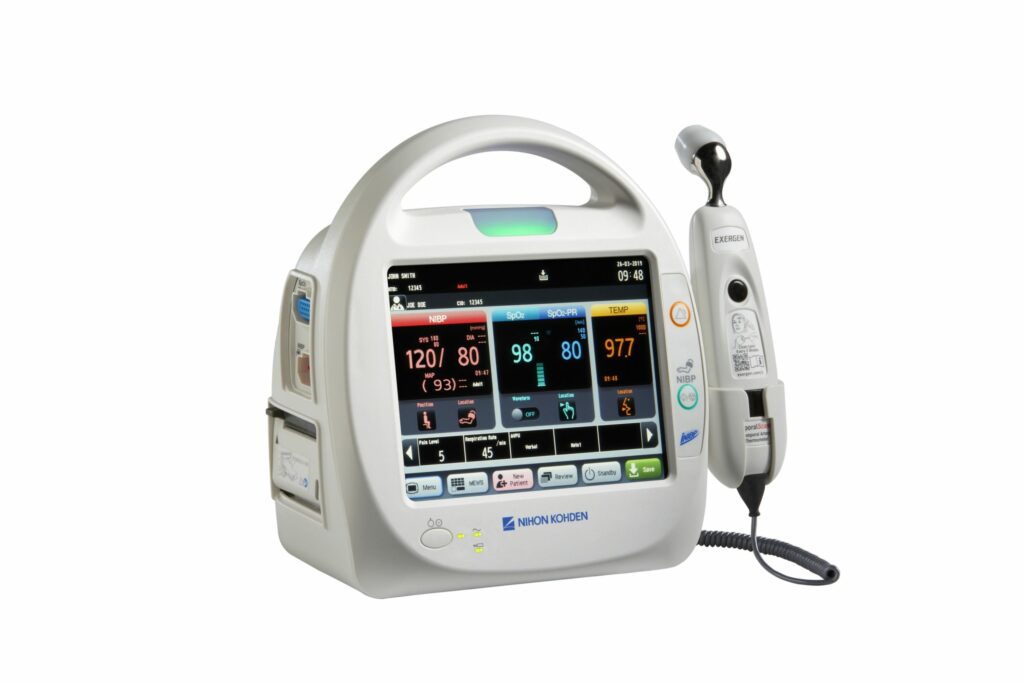
Read MoreNihon Kohden Launches Life Scope SVM-7200 Series Vital Sign Monitor, a Portable, Easy-to-Use Monitor with Customizable Early Warning Scoring
Nihon Kohden, a U.S. market leader in precision medical products and services, today announced the launch of its Life Scope® SVM-7200 Series vital signs monitor, a monitor designed for outpatient facilities and beds that traditionally are not continuously monitored. The monitor allows healthcare practitioners to quickly and easily measure three vital signs – blood oxygen,…
Read MoreIRRAflow Wins CONNECT’s “2019 Most Innovative New Product” Award
Breakthrough medical device that transforms treatment of intracranial bleeding recognized as most innovative new medical device from southern California. IRRAS AB, a global healthcare company with a comprehensive portfolio of innovative products for neurocritical care, announced today that IRRAflow has been selected as the 2019 winner of CONNECT’s Most Innovative New Product award in the medical…
Read MorePermanent Hair Dye and Straighteners May Increase Breast Cancer Risk
Scientists at the National Institutes of Health found that women who use permanent hair dye and chemical hair straighteners have a higher risk of developing breast cancer than women who don’t use these products. The study published online Dec. 4 in the International Journal of Cancer and suggests that breast cancer risk increased with more…
Read MoreGenetron Health Obtains Approval for Semiconductor-based NGS System GENETRON S5
Genetron Health, a China-based precision oncology company that covers full-cycle cancer care, has obtained regulatory approval in China to launch its GENETRON S5 for clinical use. The approval of a new desktop medium-throughput semiconductor-based next generation sequencing (NGS) system—GENETRON S5, showcases Genetron Health’s ongoing commitment to providing early screening, diagnosis and treatment recommendations, as well…
Read MoreUS Medical Innovations Receives FDA 510(k) Clearance for the Canady Plasma Smart XL-1000 Generator
US Medical Innovations, LLC (USMI), a Biomedical and Life Science subsidiary of US Patent Innovations, LLC (USPI) announced today the U.S. Food And Drug Administration (FDA) 510(k) clearance (#K192124) to market and sell the Canady Plasma® Smart XL-1000™ Electrosurgical Generator (CPSXEG) with accessories. The CPSXEG combines with a high frequency voltage to electrically enhanced plasma…
Read MoreHologic to sell Cynosure medical aesthetics business for $205m
Hologic, Inc. announced that it has entered into a definitive agreement to sell its Cynosure medical aesthetics business to an affiliate of investment funds managed by Clayton, Dubilier & Rice for a total purchase price of $205 million in cash, subject to certain closing adjustments. Net of these adjustments, Hologic expects net cash proceeds of…
Read More