Diagnostics & Healthcare News
Synaptic Medical Appoints Robert Ricker as President of US Commercialization
LAKE FOREST, Calif., October 24, 2019 – Synaptic Medical, a medical device company that focuses on developing breakthrough products for the treatment of arrhythmias and other cardiovascular diseases, today announced that Robert C. Ricker has been appointed President of US Commercialization. Mr. Ricker’s broad executive experience in the cardiovascular industry are invaluable to the future…
Read MoreAfter a Dramatic Rise, the Average U.S. Medical Radiation Doses Now Are Decreasing
The National Council on Radiation Protection and Measurements (NCRP) today issued a new report showing a 15 to 20 % reduction in diagnostic and interventional medical radiation doses to the U.S. population from 2006 to 2016. Except for computed tomography (CT) scans, most medical imaging doses are stable or decreasing. This finding is a contrast to the dramatic rise…
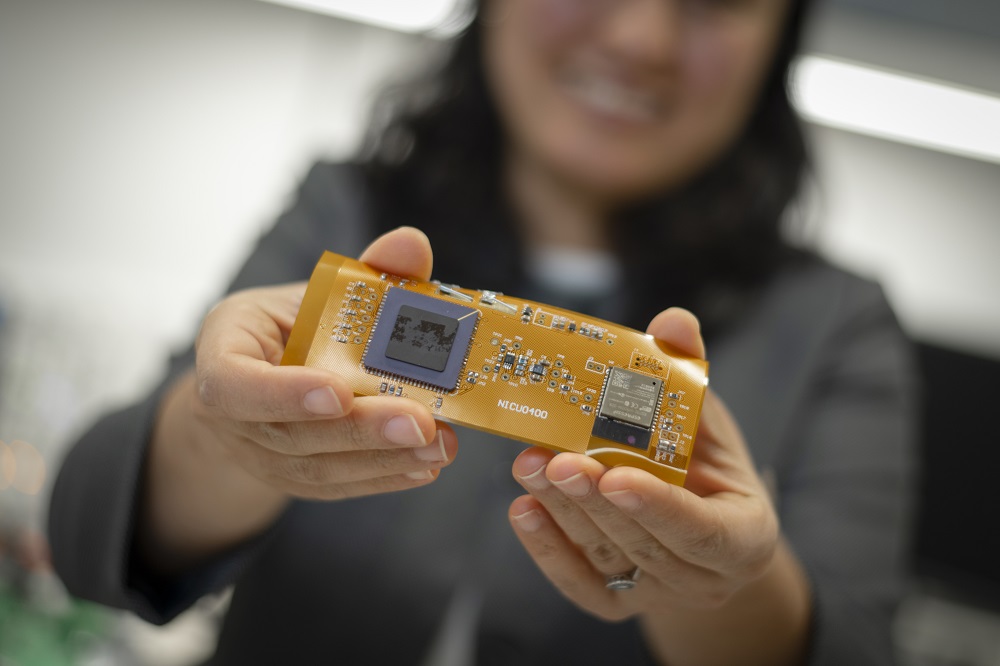
Read MoreWPI Engineers Create a Miniaturized, Wireless Oxygen Sensor for Sick Infants to Enable Parents to Take Them Home Sooner
Researchers at Worcester Polytechnic Institute (WPI) are developing a sensor the size of a Band-Aid that will measure a baby’s blood oxygen levels, a vital indication of the lungs’ effectiveness and whether the baby’s tissue is receiving adequate oxygen supply. Unlike current systems used in hospitals, this miniaturized wearable device will be flexible and stretchable,…
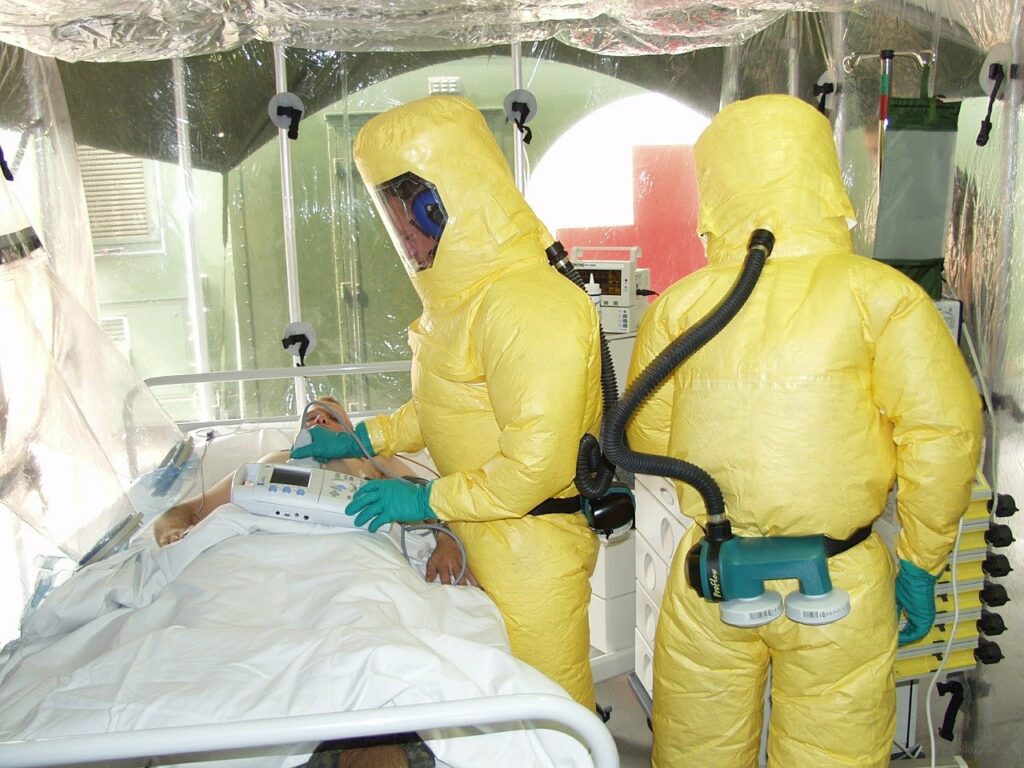
Read MoreAHF Lauds WHO’s Belated Pre-Qualification of the Ebola Vaccine
After more than four years of testing and vaccinations under compassionate use protocols, the most effective Ebola vaccine has at last received World Health Organization (WHO) pre-qualification, clearing regulatory hurdles for its wide-scale use. “AHF has been calling for an approved vaccine for more than a year—even before the current outbreak in the DRC (Democratic Republic of…
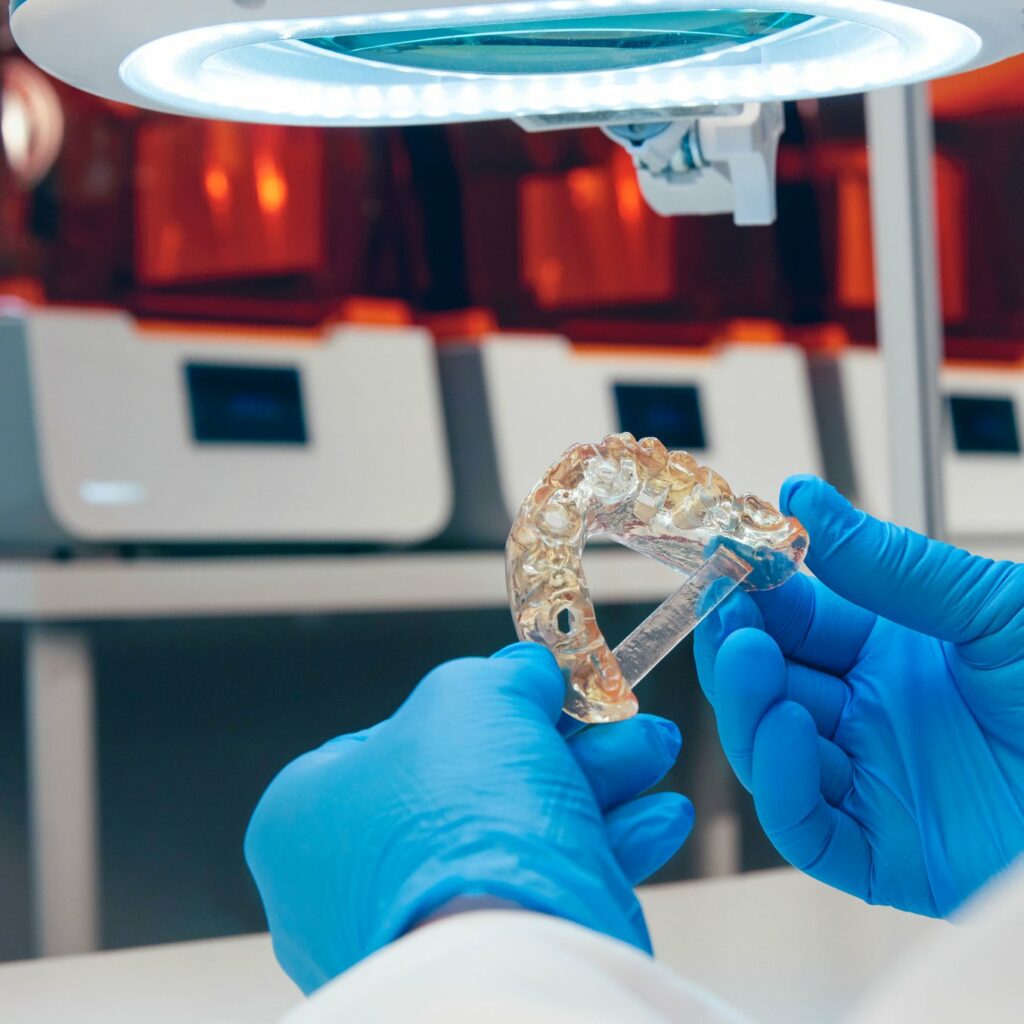
Read MoreFormlabs Launches New Dental Business Unit, Formlabs Dental, With Form 3B and New Materials Designed to Meet the Demands of the Dental Industry
Formlabs, the designer and manufacturer of powerful and accessible 3D printing systems, has today announced it is strengthening its commitment to the dental market. According to a Key Group survey, Formlabs is the most installed 3D printer for small, medium and large sized dental labs. Formlabs Dental, the company’s newly announced dental business unit, is made up of…
Read MoreAbbott Announces Robert B. Ford to Succeed Miles D. White as Chief Executive Officer on March 31, 2020
Abbott announced that Miles D. White will be stepping down as Chief Executive Officer on March 31, 2020, after a remarkable 21-year tenure, the second longest for a non-founder in today’s S&P 100. He will remain Executive Chairman of the Board. The Board has unanimously appointed Robert B. Ford, a 23-year Abbott veteran, to succeed Mr. White as…
Read MoreSonde Health Granted Foundational U.S. Patent for Use of Vocal Biomarkers in Health Assessments
Sonde Health, Inc., a leading company developing a voice-based technology platform to measure health when a person speaks, today announced that the United States Patent and Trademark Office issued U.S. Patent No. 10,475,530, a foundational patent that covers crucial aspects of Sonde’s vocal biomarker technology. The patent covers the use of Sonde’s technology to capture,…
Read MoreAirflow Sleep, a Sleep Solutions and Advocacy Company, Launches to Fight the Sleep Epidemic
Airflow Sleep, a total sleep solutions and advocacy company that empowers people to improve sleep quality and behavior, exited stealth mode today as it prepares to launch its ZO2 Sleep System for the consumer market. Each system contains a physician-designed, patented side or back sleeper pillow supporting all types of sleepers, an advanced sleep tracking…
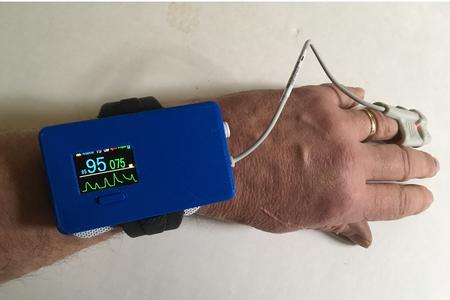
Read MoreMed-botics Wearable Device Detects Opioid Overdose, and can Provide Early Warnings to Over-sedated Patients
The FDA granted Breakthrough Device status to Med-botics, LLC, (www.med-botics.com), for its Oxalert EPOTM (Enhanced Pulse Oximeter) device. The wrist-worn arousal device is designed to prevent respiratory arrest and death from opioid overdose. FDA Guidance documents describe Breakthrough Devices as “innovative technologies that diagnose or treat life-threatening conditions more effectively than any FDA-approved products.” The FDA…
Read MoreStryker Announces Definitive Agreement to Acquire Wright Medical for $4B
Stryker announced a definitive agreement to acquire all of the issued and outstanding ordinary shares of Wright Medical Group for $30.75 per share, or a total equity value of approximately $4.0 billion and a total enterprise value of approximately $5.4 billion (including convertible notes). Wright Medical, which was founded in 1950, is a global medical…
Read More