Diagnostics & Healthcare News
Baxter Announces Acquisition of Cheetah Medical to Expand Specialized Patient Monitoring Portfolio
Baxter International Inc., a leading global medical products company, today entered into a definitive agreement to acquire Cheetah Medical, a leading provider of non-invasive hemodynamic monitoring technologies. The agreement demonstrates Baxter’s ongoing commitment to improving clinical outcomes with an established patient monitoring technology to better inform and guide clinicians’ treatment decisions. Cheetah Medical is a…
Read MoreMedtronic’s Ishrak Set to Retire, Leadership Succession Announced
Medtronic announced that Omar Ishrak, Medtronic’s Chairman and Chief Executive Officer (CEO), has announced his intention to retire as CEO on April 26, 2020, following the end of the company’s current fiscal year. As a result, the Medtronic Board of Directors announced key leadership appointments as part of its multi-year, leadership succession planning process, which…
Read MoreOneDraw A1C Needle-Free Test System FDA Cleared
Drawbridge Health, out of Menlo Park, California, won FDA clearance for its OneDraw A1C Test System, which consists of the OneDraw Blood Collection Device and the OneDraw A1C Test. Designed for use by clinicians, the disposable product is used to draw, collect, and stabilize blood to measure hemoglobin A1c (HbA1c) levels to help manage glucose…
Read MoreAllergan Recalls Textured Breast Implants Linked to Rare Type of Cancer
The FDA said today that it asked Allergan to recall its Biocell breast implants after concluding that they are six times as likely as other textured breast implants to cause an uncommon form of lymphoma. The federal safety watchdog said that nearly 84% of the 573 cases of breast implant-associated anaplastic large cell lymphoma reported worldwide…
Read MoreThese Medical Devices are now Exempt from the 25% China Tariffs
On Tuesday, the U.S. exempted several categories of medical devices from the 25% tariffs imposed on Chinese goods by the Trump administration, including surgical, radiotherapy and dental devices. The Office of the U.S. Trade Representative said the new exclusions are retroactive to July 6, 2018, when tariffs went into effect on some $34 billion in imports…
Read MoreMedtronic Recalls Some Insulin Pumps as FDA Warns They can be Hacked
The U.S. Food and Drug Administration is warning patients and health care providers that certain Medtronic MiniMed insulin pumps are being recalled due to potential cybersecurity risks and recommends that patients using these models switch their insulin pump to models that are better equipped to protect against these potential risks. To date, the FDA is not…
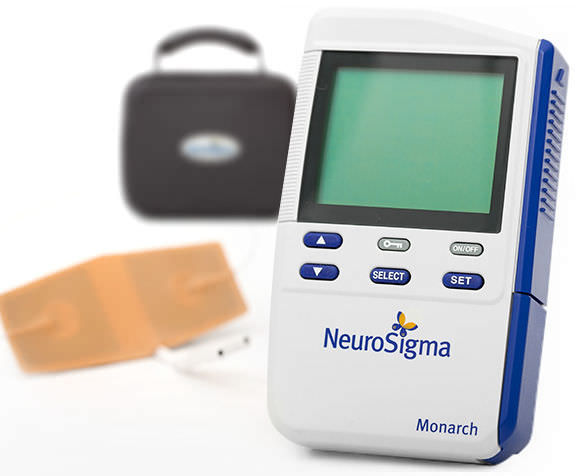
Read MoreFirst Medical Device Treatment for Childhood ADHD Cleared by FDA
NeuroSigma won de novo clearance from the FDA last week to put the first medical device for treating attention-deficit hyperactivity disorder on the U.S. market. Los Angeles-based NeuroSigma makes the Monarch eTNS system, which is designed to deliver mild electrical signals through a forehead patch to stimulate branches of the trigeminal nerve during sleep. The device won CE Mark approval…
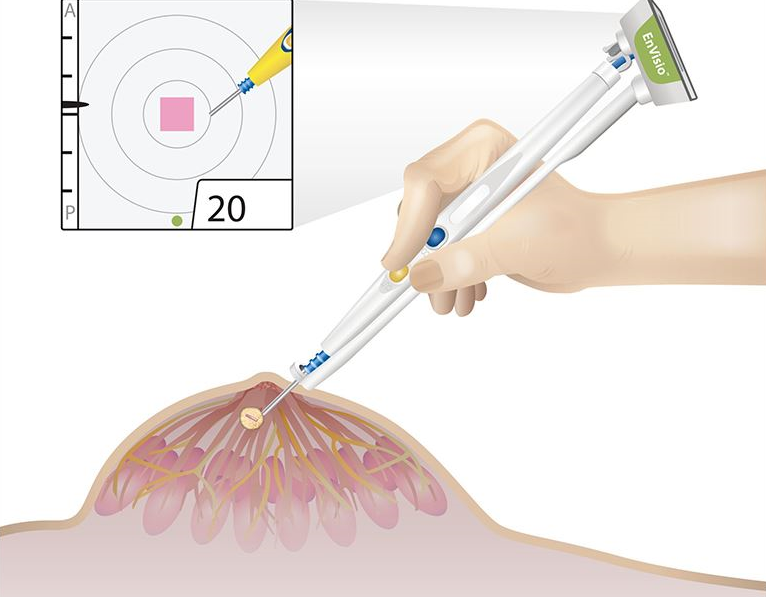
Read MoreElucent Medical Wins FDA Clearance for New Breast Surgery Navigation System
Elucent Medical, a company dedicated to developing better pathways for breast cancer care, today announced that it has received U.S. Food and Drug Administration (FDA) 510(k) clearance for the clinical use of the company’s EnVisio™ Surgical Navigation System. Placed at the time of the breast biopsy, Elucent Medical’s permanently implantable wireless SmartClip™ Soft Tissue Marker…
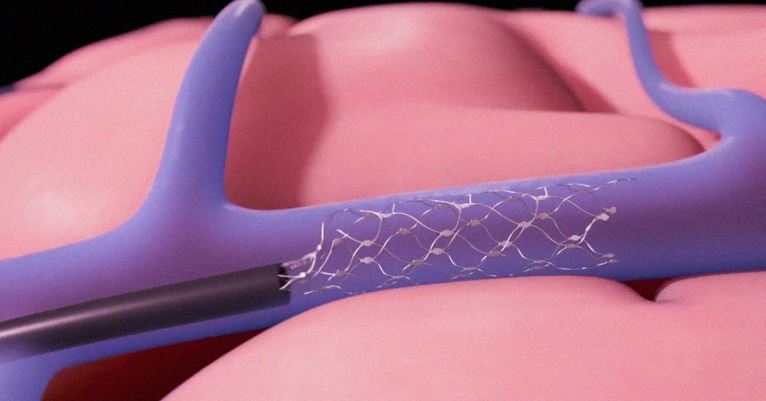
Read MoreFirst-Ever Clinical Trial Initiated for Neural Interface that Turns Thoughts into Speech
Synchron, Inc. today announced the initiation of the first clinical trial for the Stentrode™, a minimally-invasive neural interface technology being investigated for restoration of communication in people with severe paralysis. The trial will evaluate the safety of Thought-to-Text™ technology in patients, by assessing the Stentrode implant in combination with BrainOS™ software. The Stentrode is designed to record brain activity…
Read MoreItamar Medical Launches Next-Generation WatchPAT System for Home Sleep Apnea Testing
Itamar Medical Ltd., a company that develops, manufactures and markets non-invasive diagnostic medical devices for sleep apnea with a focus on the cardiology market, today announced the launch of WatchPAT 300, the next generation WatchPAT system for home sleep apnea testing. WatchPAT 300 includes several advances that are designed to enhance both patients’ WatchPAT experience…
Read More