Diagnostics & Healthcare News
FDA Clears Cytovale’s® IntelliSep® Sepsis Test, First in a New Class of Emergency Department-Focused Diagnostic Tools
Cytovale®, a medical diagnostics company focused on advancing early detection technologies to diagnose fast-moving and immune-mediated diseases, announced today that its IntelliSep® test has received U.S. Food and Drug Administration (FDA) 510(k) clearance to aid in the early detection of sepsis for the approximately 30 million adult patients with signs and symptoms of infection who present…
Read MoreMicronoma Receives FDA Breakthrough Device Designation for OncobiotaLUNG, A Novel Liquid Biopsy Assay for Lung Carcinoma Detection
Micronoma, the first biotech company offering early cancer detection with a microbiome-driven liquid biopsy platform, announced that its OncobiotaLUNG assay received the Breakthrough Device Designation from the Food and Drug Administration (FDA). As a result, Micronoma can expect continued guidance and prioritized reviews from the agency of its upcoming clinical trial and concomitant pre-market approval…
Read MoreVERO Biotech Receives FDA Approval of its Third Generation Tankless Inhaled Nitric Oxide Delivery System
VERO Biotech Inc., a commercial-stage healthcare business dedicated to neonatal intensive care and the acute care hospital community, announced FDA approval of the newest generation of its tankless inhaled nitric oxide (iNO) delivery system. The Third Generation GENOSYL® Delivery System – developed for respiratory therapists by respiratory therapists – has new features that are expected to deliver three key benefits…
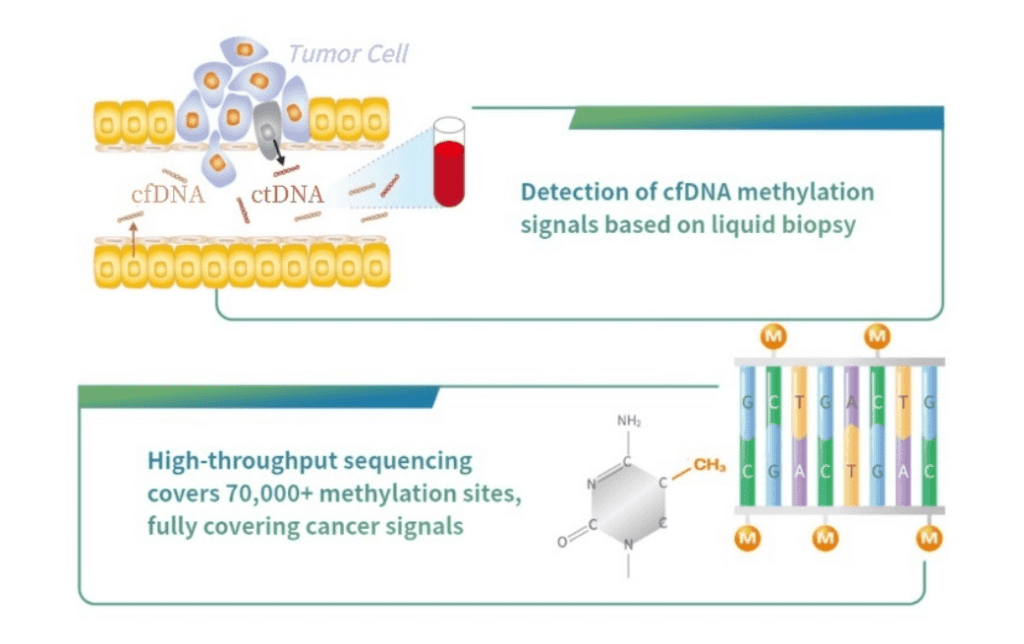
Read MoreBurning Rock Received FDA Breakthrough Device Designation for its OverC™ Multi-Cancer Detection Blood Test
Burning Rock, a company focused on the application of next generation sequencing (NGS) technology in the field of precision oncology, announced that its OverC™ Multi-Cancer Detection Blood Test (MCDBT) has been granted Breakthrough Device Designation by the US Food and Drug Administration (FDA), which is the third of its kind globally. Under the FDA’s Breakthrough…
Read MoreVisby Medical™ Receives FDA Emergency Use Authorization for Respiratory Health Test for use in CLIA waived settings
Visby Medical™ announced that it has received Emergency Use Authorization (EUA) from the U.S. Food and Drug Administration for its Respiratory Health point of care test. The Visby Medical Respiratory Health Test is a fast, polymerase chain reaction (PCR) device that detects and differentiates between upper respiratory infections caused by Influenza (Flu) A & B, and SARS-CoV-2 (COVID-19). Visby Medical has…
Read MorePennsylvania-based Highmark Inc. Joins Theranica’s Coverage with Evidence Program for Nerivio® Drug-Free Migraine Treatment Device
Theranica, a prescribed digital therapeutics company developing advanced neuromodulation devices for migraine and other pain conditions, announced that Highmark Inc. (Highmark), one of America’s leading health insurance organizations and an independent licensee of the Blue Cross Blue Shield Association, will conduct a targeted Coverage with Evidence program with the goal of providing its members with affordable…
Read MorePromising Remote Patient Monitoring solution, Polso, Achieves 1st FDA Clearance
ChroniSense Medical Ltd. took a step towards its vision of transforming chronic care management with Polso™, the company’s Remote Patient Monitoring (RPM) solution, achieving 510(k) clearance from the U.S. Food and Drug Administration (FDA). Initial indications include monitoring of blood oxygen saturation (SpO2), pulse rate and respiration rate. “FDA clearance is a significant milestone towards…
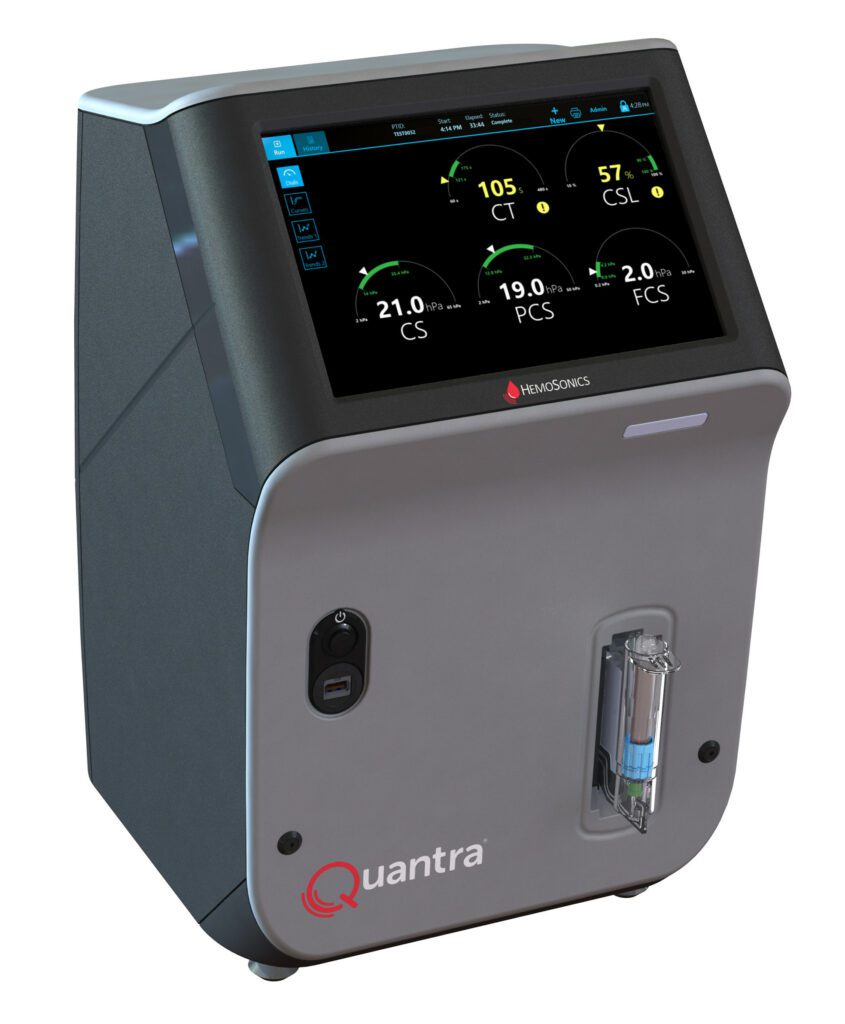
Read MoreHemosonics Awared FDA 510(k) Clearance for Quantra® Hemostasis System with QSTAT® Cartridge
HemoSonics, LLC, a leading medical device company delivering individualized diagnostic solutions for Patient Blood Management (PBM), announced that it has received 510(k) market clearance from the U.S. Food and Drug Administration (FDA) for the Quantra Hemostasis System with QStat Cartridge. “The Quantra Hemostasis System with QStat Cartridge is breaking new ground and leading innovation in the…
Read MoreElemental Machines Raises $41M in Series B Funding to Fuel Growth
Elemental Machines, a developer of the LabOps Intelligence Platform for labs around the world, has raised $41 million in a Series B funding round led by Sageview Capital and co-led by Omega Venture Partners, with continued participation from Gutbrain Ventures and Digitalis Ventures. “The support we have received from Sageview Capital, Omega Venture Partners, and our existing…
Read MoreEyenuk secures $26 Million Series A funding to accelerate global access to AI-powered eye-screening technology
Eyenuk, Inc., a global artificial intelligence (AI) digital health company and the leader in real-world applications for AI Eye Screening™ and AI Predictive Biomarkers™, announced it has secured $26 million in a Series A financing round, bringing the Company’s total funding to over $43 million. The capital raise was led by AXA IM Alts and…
Read More