Imaging Visualization & Navigation
FDA Awards HistoSonics Clearance of its First-of-a-Kind Edison® Histotripsy System
HistoSonics®, (www.histosonics.com), the manufacturer of the Edison® System and novel histotripsy therapy platforms, announced today the marketing authorization of its “Breakthrough” platform via the U.S. Food and Drug Administration’s (FDA) De Novo Classification Request process, a rigorous pre-market review pathway for medical devices with no existing predicate. Marketing authorization makes Edison the first and only histotripsy…
Read MoreSaluda Medical Receives FDA Approval for Evoke® System MRI Labeling
Saluda Medical, Inc. (“Saluda Medical”), a global medical device company revolutionizing the field of neuromodulation with an emerging portfolio of therapies driven by advanced closed-loop technologies, today announced that the U.S. Food and Drug Administration (FDA) has approved MRI conditional labeling for the Evoke® System, the first and only precision, dose-control spinal cord stimulation (SCS) therapy…
Read MoreSpectraWAVE Secures 510(k) Clearance to Add Saline Imaging and Expanded Artificial Intelligence Features to the HyperVue™ Imaging System
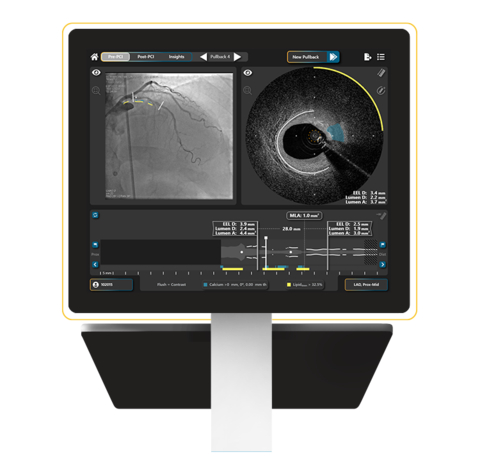
SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced U.S. Food and Drug Administration (FDA) 510(k) clearance for product enhancements to the HyperVue™ Imaging System. The intravascular imaging system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of…
Read MoreZeta Surgical’s Mixed Reality Navigation System Receives FDA Clearance
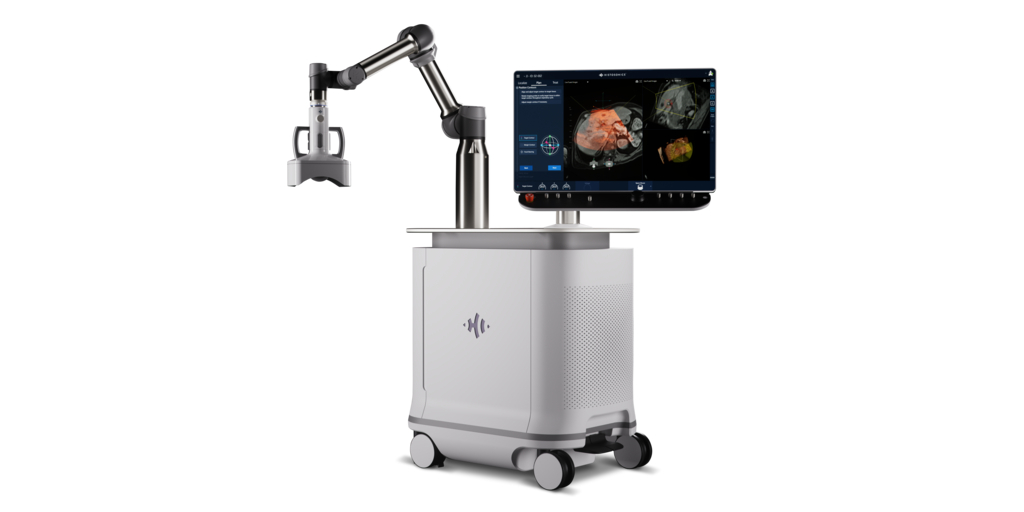
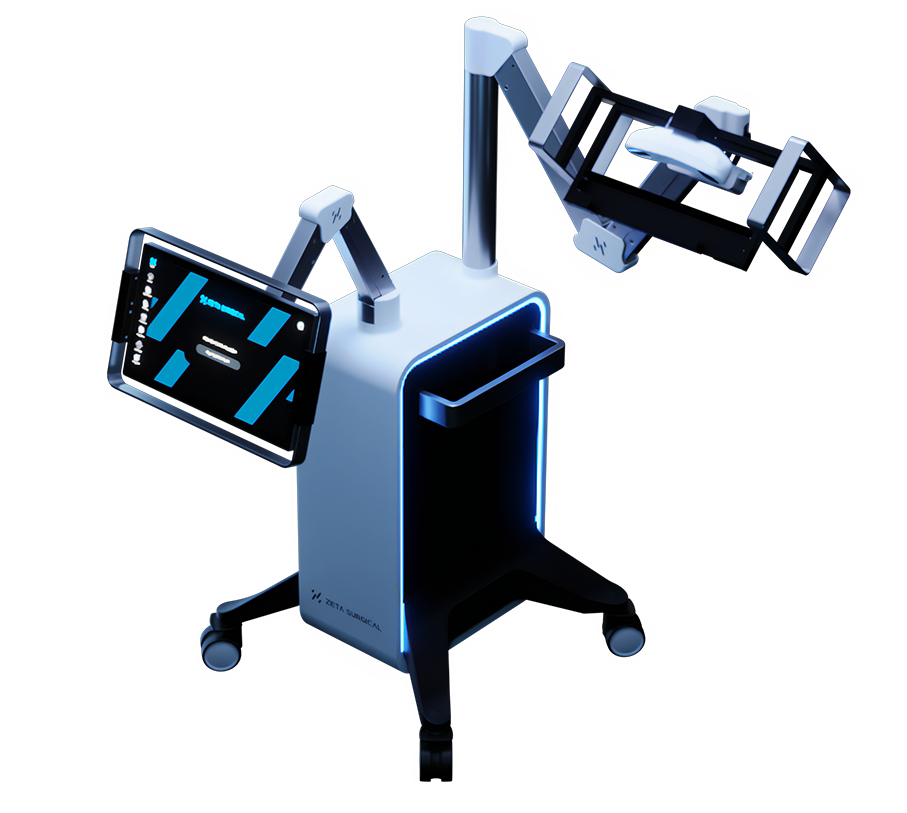
Zeta Surgical, a surgical robotics and mixed reality company, announced today that the U.S. Food and Drug Administration has cleared the Zeta Cranial Navigation System, its mixed reality surgical navigation system. The Zeta Cranial Navigation System is a mixed-reality navigation system for neurosurgery that provides surgeons with “GPS-like” guidance with millimetric accuracy in real time. Zeta’s…
Read MoreTechsomed Announces FDA Clearance for Ablation Treatment Planning and Confirmation Software
Techsomed Ltd., a medical software innovator dedicated to enhancing clinical impact in ablation therapy, announced today that it has received 510(k) clearance from the USA Food and Drug Administration (FDA) for its VisAble.IO software intended to assist physicians in planning liver ablation procedures, and confirming ablation zones, with the goal of increasing treatment precision. Ablation…
Read MoreAxial3D Receives FDA Clearance for Axial3D INSIGHT™ Medical Image Segmentation Platform
Axial3D, a leader in medical segmentation and 3D solutions, today announced that it is the first to receive FDA clearance for an automated, AI-driven, cloud-based segmentation platform for orthopedic trauma, orthopedic, maxillofacial, and cardiovascular applications. This achievement is the second FDA clearance Axial3D has received for its segmentation platform, INSIGHT™, and a significant milestone for…
Read MoreRIVANNA receives $30.5 million from BARDA to advance the Accuro XV musculoskeletal imaging system
RIVANNA®, developers of imaging-based medical solutions, announced that they have received funding totaling $30.5 million over 39 months from early execution of an option by the Biomedical Advanced Research and Development Authority (BARDA), part of the Administration for Strategic Preparedness and Response (ASPR) within the U.S. Department of Health and Human Services (HHS). The funding will support…
Read MoreMediView Receives FDA 510(k) Clearance for XR90 Augmented Reality-Based Visualization and Navigation Platform
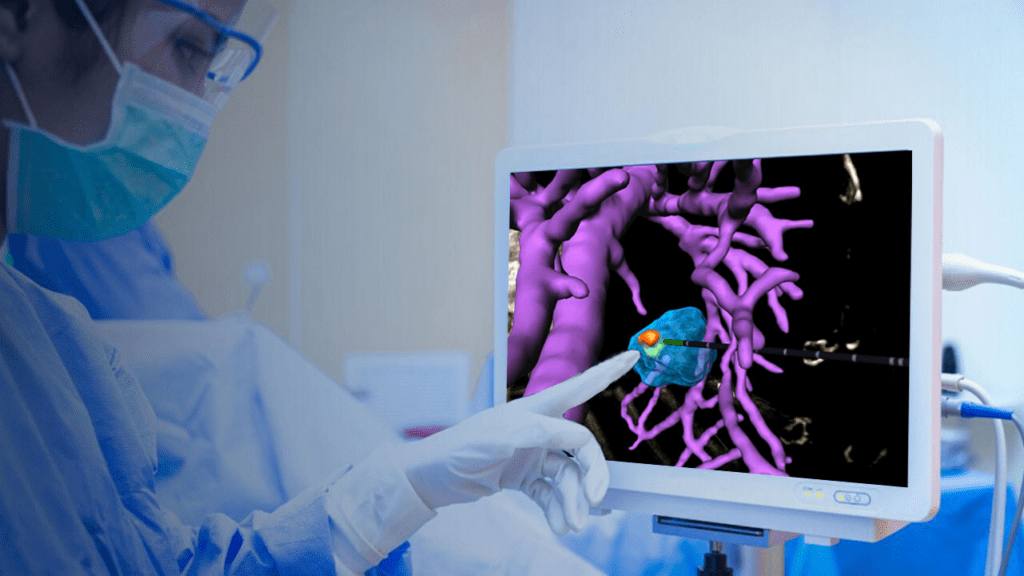
MediView XR, Inc., a leading clinical augmented reality med-tech company, announced today it received 510(k) clearance from the U.S. Food and Drug Administration (“FDA”) for its XR90 augmented reality-based surgical visualization and navigation platform. XR90 is intended to be used adjunctively for minimally invasive ultrasound and CT guided needle-based procedures for soft tissue and bone.…
Read MoreWaters Takes Targeted, Quantitative Imaging to the Next Level with New DESI Source for the Xevo TQ Absolute System
American Society for Mass Spectrometry (ASMS) — Waters Corporation (NYSE:WAT) today launched the industry’s first targeted imaging mass spectrometer based on its Xevo™ TQ Absolute tandem quadrupole mass spectrometer, the most sensitive and compact mass spectrometer in its class. The new instrument combines the Waters™ DESI XS source with the Xevo TQ Absolute system and is five times more sensitive and five…
Read MoreOrlucent Receives FDA Breakthrough Device Designation for Handheld Mole Imaging System
Orlucent, Inc., a company focused on clinical assessment of suspicious moles, today announced the U.S. Food and Drug Administration (FDA) has granted Breakthrough Device Designation for the company’s Orlucent® Skin Fluorescence Imaging System. The Orlucent system is a handheld point-of-care molecular-based imaging system designed to noninvasively identify and clinically assess the presence of biological tissue…
Read More