Imaging Visualization & Navigation
Koning Sees Surge in Orders Following Recent Installations at Prominent Hospitals and Attendance at the Society of Breast Imaging Symposium
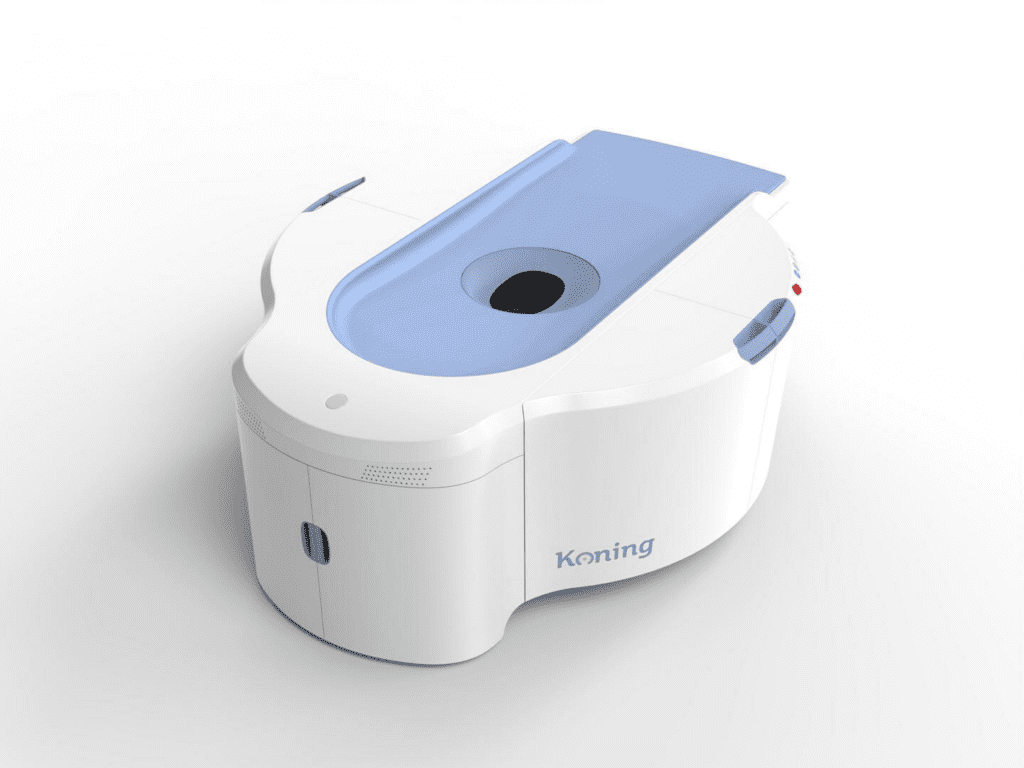
Koning Corporation, the leading manufacturer of breast computed tomography (CT) systems, announced today that it has received the largest pipeline of orders in its history. The company has seen a surge in demand for its innovative Koning Vera Breast CT system from healthcare providers around the world, as they seek to improve breast cancer detection…
Read MoreInnoblative Receives U.S. FDA Breakthrough Device Designation for its SIRA RFA Electrosurgical Device
Innoblative Designs, Inc. (Innoblative), a private medical device company addressing clinical unmet needs for patients with breast cancer, announced that it received Breakthrough Device Designation from the U.S. Food and Drug Administration (FDA) for the company’s SIRA™ RFA Electrosurgical Device (SIRA). The SIRA device is intended for use in breast cancer patients undergoing BCS, commonly…
Read MoreRapidAI Receives First and Only FDA 510(k) Clearance of Non-Contrast CT Imaging Product to Accelerate Acute Stroke Triage
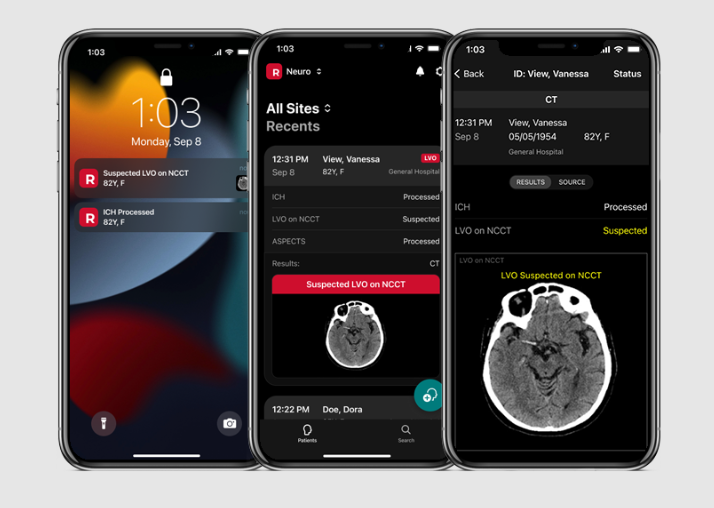
RapidAI, the global leader in neurovascular and vascular AI-enhanced clinical decision support and patient workflow, today announced it has received FDA 510(k) clearance for Rapid NCCT Stroke – a major addition to the RapidAI suite of non-contrast based solutions for stroke and trauma care, and the first and only FDA cleared medical device to detect suspected intracranial…
Read MorePrecision Optics Appoints Mahesh Lawande as Chief Operating Officer
Precision Optics Corporation, Inc. (NASDAQ: POCI), a leading designer and manufacturer of advanced optical instruments for the medical and defense industries, today announced the appointment of medical device and aerospace/defense industry manufacturing veteran, Mahesh Lawande, as the Company’s Chief Operating Officer effective April 24, 2023. In the newly created role, Lawande will lead Precision Optics operations team, including…
Read MoreSentiAR Announces Close of $8.5 million Series B Financing
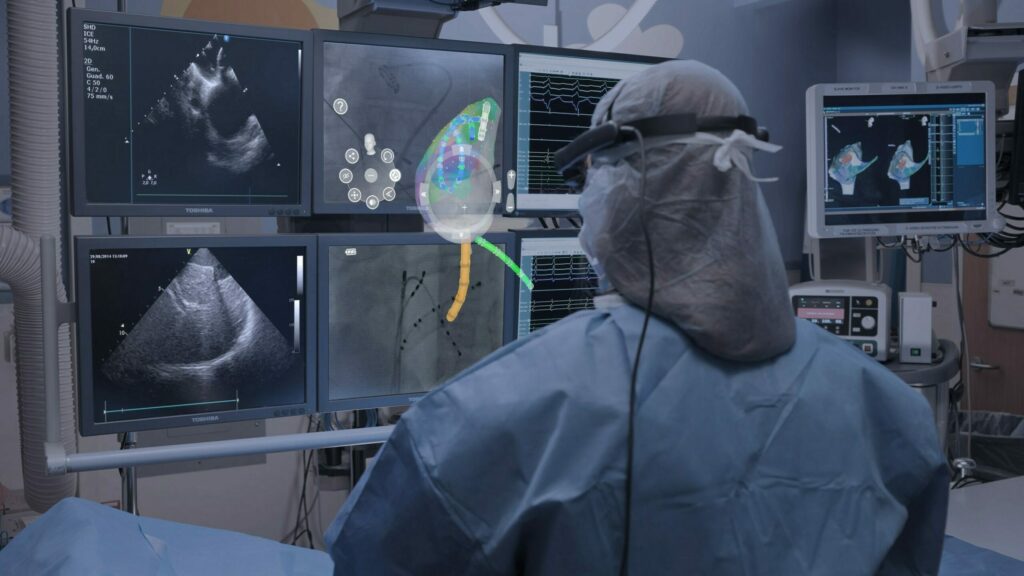
SentiAR, Inc. a St. Louis, Missouri based pioneer in using Augmented Reality (AR) visualization technology for medical procedures, has closed an $8.5 million Series B financing. The financing was led by cultivate (MD) Ventures and joined by MedVenture Partners alongside several insider investors, including TechWald Holding, VCapital, QRM Capital, and Harmonix Fund. The funding will allow the company to launch…
Read MoreFemDx Medsystems, Inc. Receives FDA Clearance For Its FalloView™ Falloposcope
FemDx Medsystems, Inc., a women’s health startup based in Santa Clara, CA announces the company has received U.S. Food and Drug Administration (FDA) 510(k) clearance for its FalloView™ device. According to Ashlee Francis, CEO of FemDx Medsystems, Inc., the device is the first totally disposable 1.2mm diameter falloposcope incorporating a CMOS chip endoscope to evaluate…
Read MoreFDA Expands Indications for Use of FibroScan® for Comprehensive Liver Management
The U.S. Food and Drug Administration (FDA) has cleared expanded Indications for Use for screening with FibroScan®, the non-invasive liver management technology by Echosens. The update furthers FibroScan® accessibility to more people, enabling physicians to identify those at risk of suffering adverse liver outcomes and connecting them with preventative care and treatment options. The new clearance removes…
Read MoreLumicell Submits New Drug Application for LUMISIGHT™ Optical Imaging Agent to U.S. FDA for Intraoperative Breast Cancer Detection and Removal
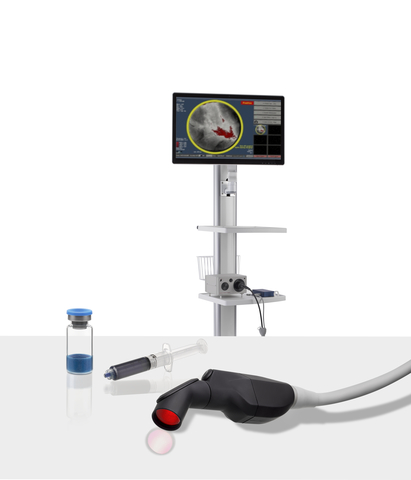
Lumicell, Inc., a privately held company focused on innovative fluorescence-guided imaging technologies for cancer surgery, today announced a New Drug Application (NDA) for its LUMISIGHT™ Optical Imaging Agent has been submitted to the U.S. Food and Drug Administration (FDA). LUMISIGHT is intended for use with the Lumicell™ Direct Visualization System (DVS), an investigational system designed…
Read MoreSpectraWAVE Secures 510(k) Clearance of HyperVue™ Intravascular Imaging System
SpectraWAVE, Inc., a medical imaging company focused on improving the treatment and outcomes for patients with coronary artery disease (CAD), today announced Food and Drug Administration (FDA) 510(k) clearance of their flagship intravascular imaging system, HyperVue™. The system combines next-generation DeepOCT™ images and near infrared spectroscopy (NIRS) with state-of-the-art ease of use to support physicians…
Read MoreHyperfine, Inc. Announces FDA Clearance for Improved AI-Powered Software and Expanded Field of View for the Swoop® Portable MR Imaging® System
Hyperfine, Inc., the groundbreaking medical device company that created the Swoop® system, the world’s first FDA-cleared portable magnetic resonance imaging (MRI) device for imaging of the brain, today announced the U.S. Food and Drug Administration (FDA) 510(k) clearance and launch of the company’s upgraded AI-powered software. The most recently cleared Swoop® software improves the image…
Read More