Imaging Visualization & Navigation
FDA Clears New Disposable Fog-Free Articulating 5mm Laparoscope
Xenocor, Inc. announced the FDA cleared the new Xenocor Disposable 5mm Articulating Laparoscope for minimally invasive abdominal and thoracic surgery. The Xenoscope 5mm articulating disposable laparoscope is designed to improve image quality, reduce fog, lower hospital costs, and reduce bio-hazard risk for the patient and staff. “Disposable laparoscopes can reduce hospital costs and prevent cross-contamination between…
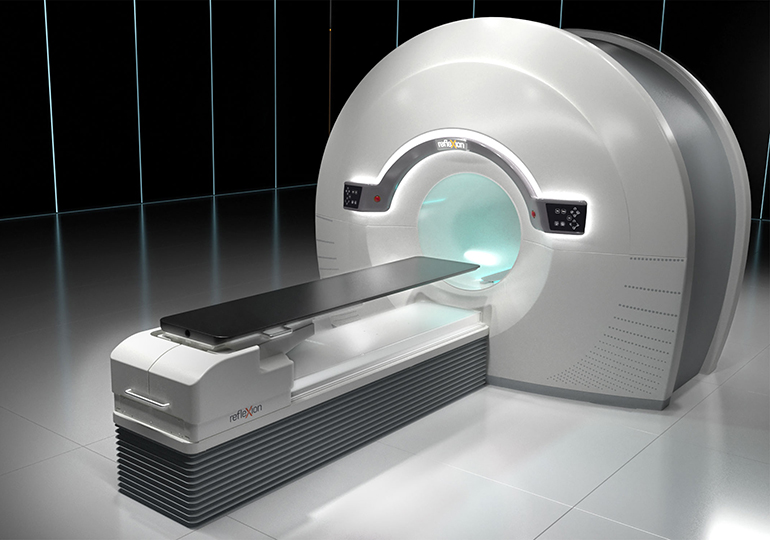
Read MoreFDA Clearance Brings RefleXion Closer to Expanding Cancer Treatment Market
RefleXion Medical, a therapeutic oncology company pioneering the use of biology-guided radiotherapy (BgRT)* to treat all stages of cancer today announced it has received marketing clearance from the U.S. Food & Drug Administration (FDA) for stereotactic body radiotherapy (SBRT), stereotactic radiosurgery (SRS) and intensity modulated radiotherapy (IMRT) for its RefleXion™ X1 machine. “We are at the…
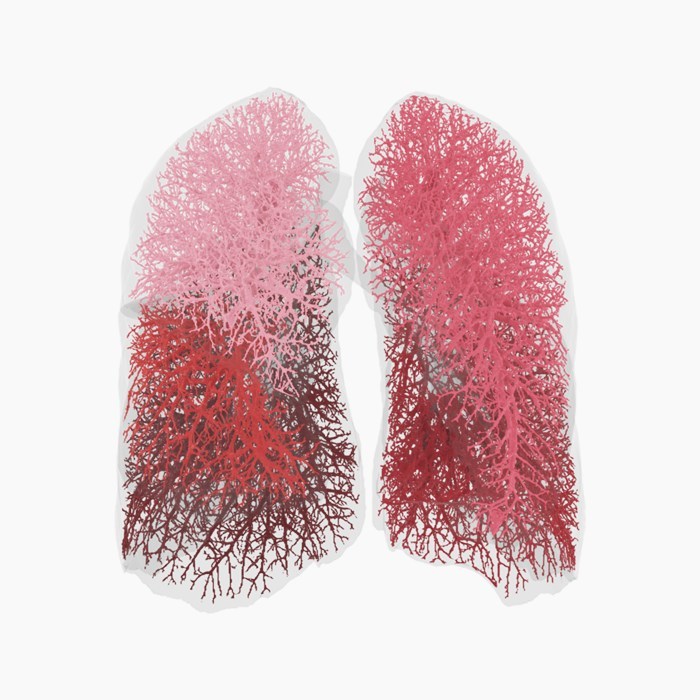
Read MoreFDA Clears Fluidda’s Broncholab Platform for Use in Clinical Practice
The US Food and Drug Administration provided market clearance to Fluidda for its Broncholab platform. Broncholab provides a number of Functional Respiratory Imaging (FRI) parameters to physicians via an online platform to assist in diagnosing and monitoring of respiratory diseases. The severity of respiratory diseases is often underestimated by conventional lung function tests, such as…
Read MoreNeuroPace RNS System Receives FDA Approval for MRI Labeling, Allowing Thousands More Patients to Benefit from Personalized, Data-Driven Epilepsy Treatment
NeuroPace, Inc., a Silicon Valley-based medical technology company, today announced that its RNS® System has received U.S. Food and Drug Administration (FDA) approval of MRI labeling for the RNS System, expanding treatment options for the approximately one million patients in the United States living with seizures that do not respond to medication. Individuals with focal onset…
Read MoreNanox Signs Agreement With The Gateway Group for the Deployment of 1,000 Nanox.ARC Units Across Australia, New Zealand, and Norway
NANO-X IMAGING LTD (www.nanox.vision) (“Nanox” or the “Company”), an innovative medical imaging technology company, announces it has secured an exclusive distribution deal with The Gateway Group (“Gateway”), one of Australia’s largest independent product distributors including health, wellness, medical supplies and devices. The agreement has an initial term of three years and is renewable for an additional term of…
Read MoreArizona Startup Emagine Solutions Technology Receives FDA 510(k) Clearance of its VistaScan Mobile Ultrasound
Emagine Solutions Technology, an award-winning medical software device company located in Tucson, announced that it has received clearance from the U.S. Food & Drug Administration (“FDA”) to market its VistaScan mobile ultrasound platform. The VistaScan platform transforms a clinician’s cell phone or tablet into a portable ultrasound solution. The system consists of compatible FDA cleared ultrasound probes…
Read MoreWaveGuide Launches World’s First Portable NMR Device: the WaveGuide Formµla
WaveGuide Corporation, an innovator of portable micro NMR (Nuclear Magnetic Resonance) instruments, today launched its new WaveGuide Formµla™, the world’s only battery-powered, compact scientific instrument that performs rapid screening and diagnostics of solid and liquid substances spanning an array of markets and applications. The WaveGuide Formµla™ micro NMR delivers performance as good or better than…
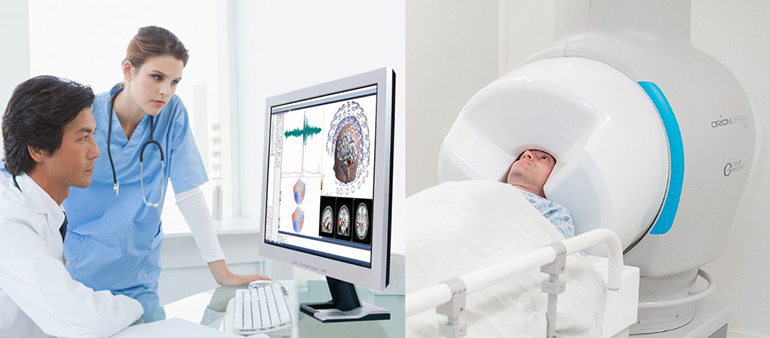
Read MoreCompumedics Announces Milestone US FDA Approval for the Orion Lifespan MEG
Compumedics Limited is pleased to announce that on February 14, 2020, it received 510(K) clearance from the US Food and Drug Administration (FDA) for its Orion LifeSpan™ Magnetoencephalography (MEG) single Dewar system. This news follows the successful installation and first phase commissioning of the single Dewar Orion LifeSpan™ MEG at Barrow Neurological Institute (BNI) in Phoenix,…
Read MoreWorld’s First Bedside MRI System Receives FDA 510(k) Clearance
Hyperfine Research, Inc. announced today that it has received US Food & Drug Administration 510(k) clearance for the world’s first bedside Magnetic Resonance Imaging (MRI) system, clearing the way for device shipments this summer. The Hyperfine system is 20X lower cost, 35X lower power consumption, and 10X lower weight than today’s fixed conventional MRI systems.…
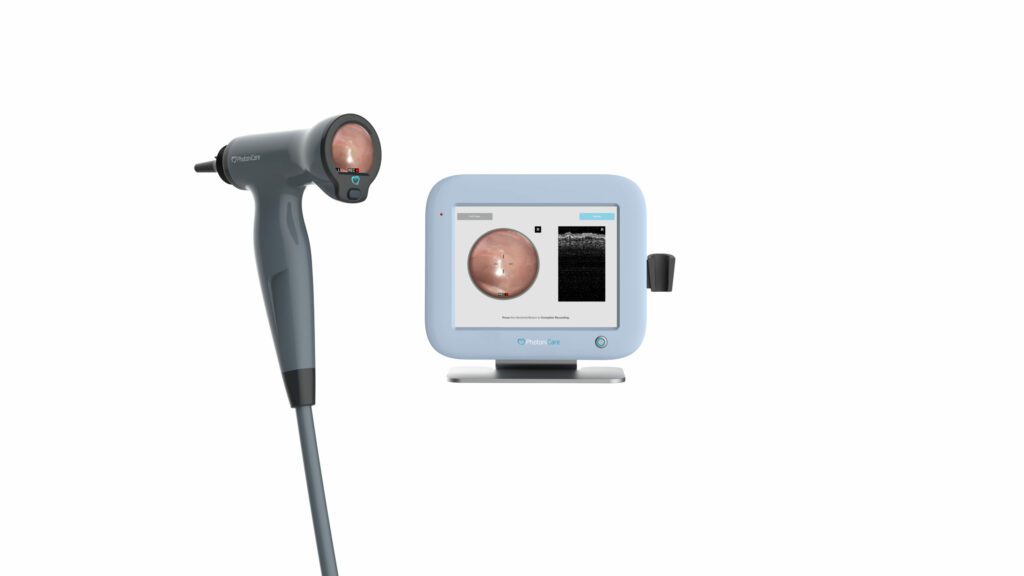
Read MorePhotoniCare Announces FDA Clearance for First-In-Class Technology for Imaging the Ear
PhotoniCare, Inc., a company dedicated to revolutionizing healthcare by providing physicians with better diagnostic tools, today announced that the U.S. Food & Drug Administration (FDA) has granted 510(k) clearance for its TOMi™ Scope for non-invasive imaging of the middle ear. Using optical coherence tomography (OCT) high resolution depth imaging, TOMi Scope helps to determine the presence or absence…
Read More