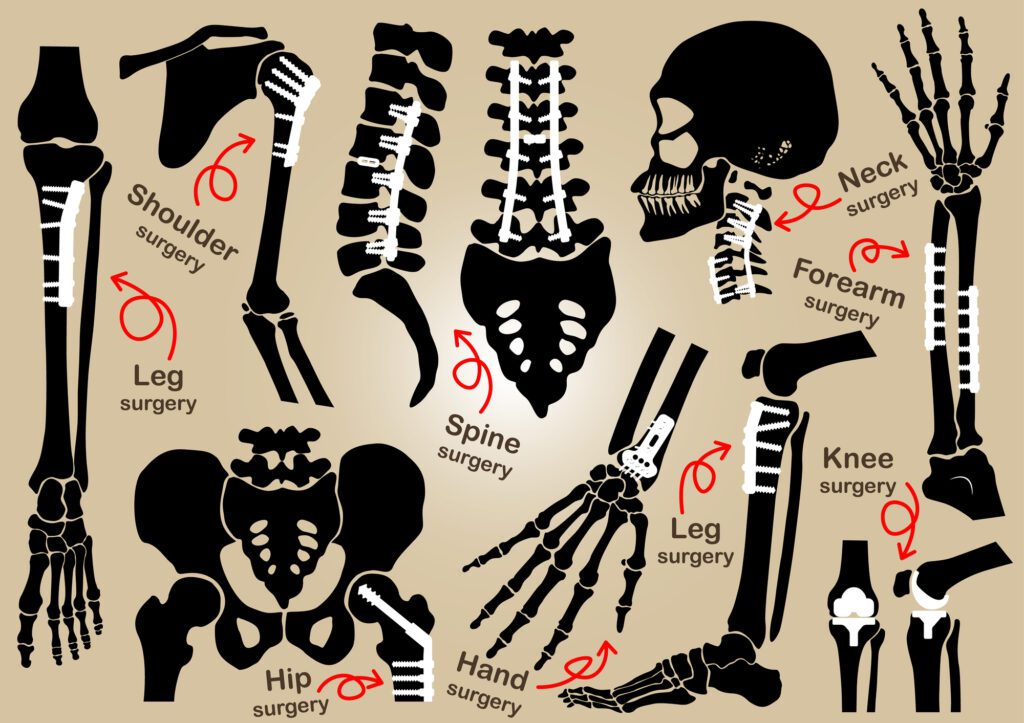
Orthopedics and Spine
FDA Clears First Nanotechnology PEEK Devices for Spinal Intervertebral Fusion
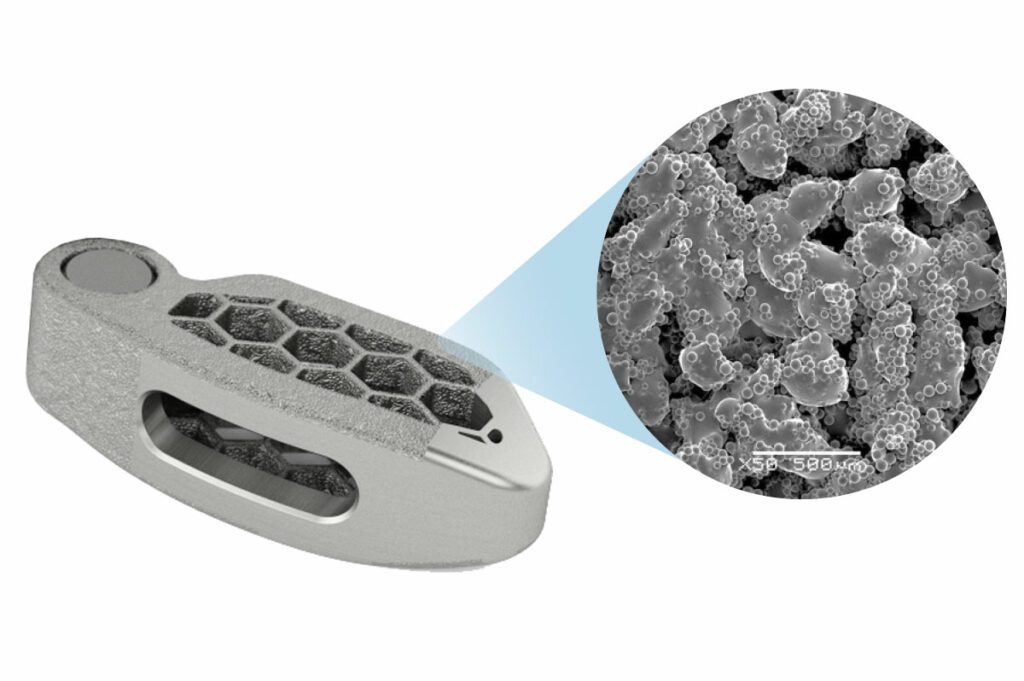
Vallum Corporation, a medical device company, today announced that it has received clearance from the U.S. Food and Drug Administration (FDA) to market a polyetheretherketone (PEEK) spinal interbody fusion device with a PEEKplus® nanotextured surface created by Vallum’s proprietary and patented Accelerated Neutral Atom Beam (ANAB) technology. PEEKplus® is the first and only FDA-cleared nanotextured…
Read MoreMedtronic Receives Expanded Indication for Kyphon HV-R Bone Cement
Medtronic seems to be on a roll when it comes to innovation. The Dublin-based company has received the greenlight from FDA for an expanded indication of the Kyphon HV-R Bone Cement. Under the new indication, Medtronic can market Kyphon for the fixation of pathological fractures of the sacral vertebral body using sacral vertebroplasty or sacroplasty.…
Read MoreScammers Trick Seniors with ‘Free’ Medical Devices, Then Bill Medicare
Nancy DeLozier looked at the array of medical equipment on the floor of her living room and called it junk. The 84-year-old Northwest Side woman said she received the items in the mail but neither wants nor needs them. One shipment, from February 2017, included a back brace and knee braces. A second shipment, which…
Read More3D Printed Bone Segments for Foot and Ankle Fixation Receive 510(k) Clearance
Additive Orthopaedics, LLC., the leader in 3D printed orthopaedic foot and ankle devices, today announced that is has received FDA 510(k) clearance for its Patient Specific 3D Printed Bone Segments, to address internal bone fixation in the ankle and foot. According to Greg Kowalczyk, President of Additive Orthopaedics, “This is a tremendous milestone for orthopaedics and…
Read MoreMedtronic’s New 3D Printed Technique to Enhance Surface Textures for Spinal Surgery Implants
DUBLIN and NEW ORLEANS – May 1, 2018 – Medtronic plc (NYSE: MDT) today announced the launch of a titanium 3D printed platform – TiONIC(TM) Technology – that enables more complex designs and integrated surface technologies for spine surgery implants. The announcement was made during the American Association of Neurological Surgeons (AANS) annual meeting in New…
Read MoreFormer Stryker Engineer Pleads Guilty to Accepting Bribe
Daniel Lawrynowicz, a former Stryker Corporation engineering director, pled guilty on April 3, 2018 to accepting a $75,000 bribe for steering business to a New York metallurgy firm. According to the Department of Justice (DoJ), Lawrynowicz, 48, pleaded guilty before U.S. District Judge Madeline Cox Arleo in Newark federal court to an information charging him…
Read MoreFDA Clears Continuous Compression Extremity Device
San Jose-based Panther Orthopedics, Inc.’s, flexible extremity joint fixation device has been cleared by the FDA for sale in the U.S. The Puma System is indicated for syndesmosis fixation, hallux valgus reconstruction, and tarsometatarsal fixation. While the FDA action is a clearance, meaning the device was essentially equivalent to a previously approved device, the company…
Read More3D Printing Promises a Revolution in Orthopaedic Device Manufacturing
FDA guidance is a first step 3D printing promises to be a revolution in orthopaedic device manufacturing. In 3D printing, parts are built up layer-by-layer by adding to a workpiece using a variety of materials and energy sources. 3D printing is more technically called additive manufacturing (AM) to distinguish it from traditional machining, which “subtracts”…
Read More19 Spine and Orthopedic Medical Device Companies to Watch in 2018
2017 was filled with mergers, acquisitions, and FDA approvals, and the momentum has not seemed to stop. Here are 19 spine and orthopedic devices companies to watch in 2018 based on their success in 2017. Alphatec (Carlsbad, Calif.) After making leadership changes this year, Alphatec expects to see around $101.4 million in full-year 2017 revenue,…
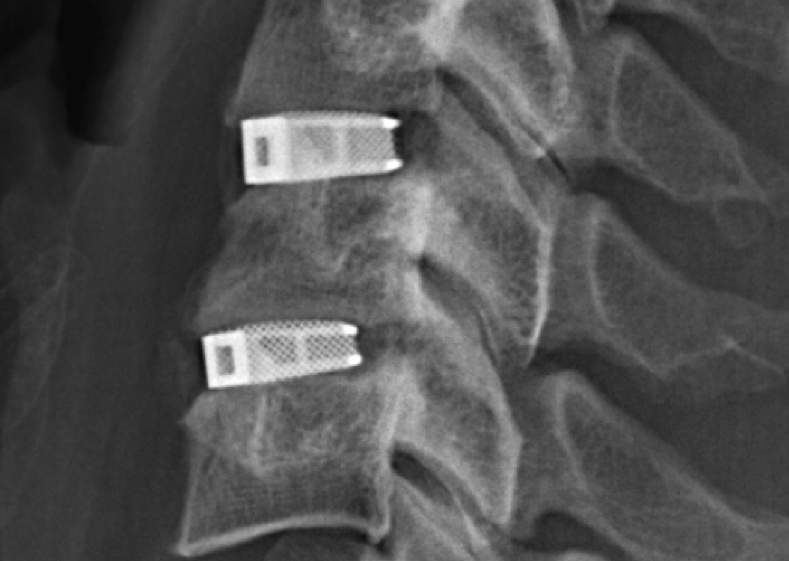
Read MoreFDA clears “first ever” 3D printed spine implant to treat of multiple injuries
Metal additive manufacturing has hit on a clear niche within the healthcare industry for producing small and complex medical grade implants at scale. Many 3D printing companies working within medicine have a portfolio of devices for the purpose, typically including a sample of 3D printed cages used to support the spine. However, in what is tipped to be…
Read More