Orthopedics and Spine
FDA Clears Life Spine Anterior Lumbar Spacer System
Life Spine, a medical device company that designs, develops, manufactures and markets products for the surgical treatment of spinal disorders, announced today that it has received clearance from the U.S. Food & Drug Administration (FDA) to market the PLATEAU-A Ti Anterior Lumbar Spacer System. “With the increased usage of anterior column reconstruction, PLATEAU-A Ti fills…
Read MoreFX Solutions Receives FDA 510k Clearances for Titanium Nitride Coated Humeral Heads and Glenospheres
FX received 510k clearances for their TiN (Titanium Nitride) Coated Humeral Heads and Glenospheres. The new addition of TiN coated humeral heads and glenospheres adds unique-to-market prostheses to the FX portfolio. The TiN coated humeral heads and glenospheres are identical to the current humeral heads and glenospheres that we offer but with the TiN coating applied. The TiN coated…
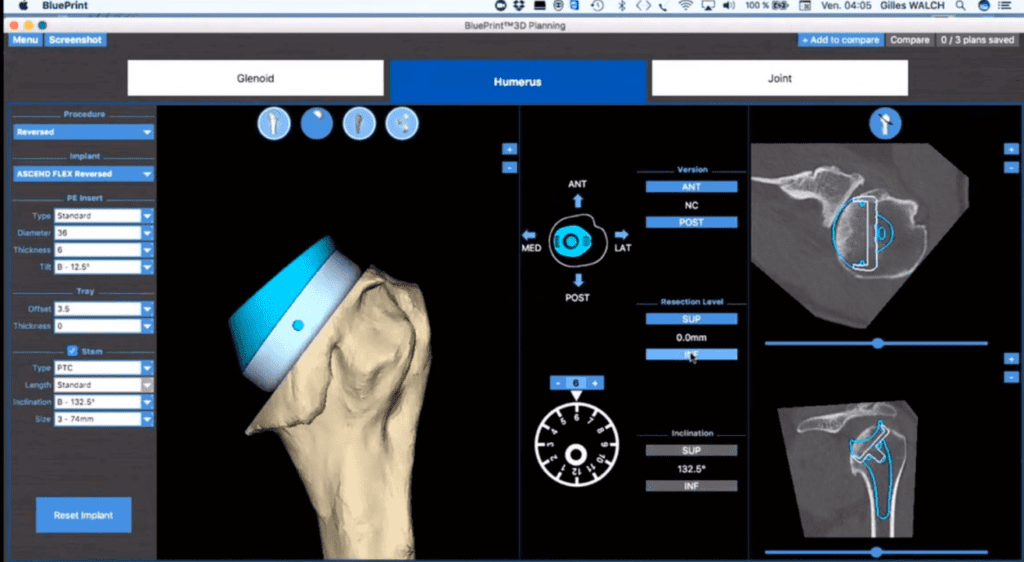
Read MoreMayo Clinic performs first shoulder arthroplasty with Wright Medical mixed reality tech
Wright Medical Group N.V. (NASDAQ: WMGI) today announced that the first shoulder arthroplasty procedure was performed using groundbreaking BLUEPRINT Mixed Reality Technology at Mayo Clinic’s campus in Rochester, Minnesota. Joaquin Sanchez-Sotelo, M.D., Ph.D, performed the procedure utilizing BLUEPRINT OR Visualization Mixed Reality software, which provides a 3-D holographic view of the patient’s pre-operative plan. Robert Palmisano, president and chief executive…
Read MoreMedtronic to Acquire Medicrea
Medtronic plc (NYSE:MDT), a global leader in medical technology, and Medicrea, a pioneer in the transformation of spinal surgery through artificial intelligence, predictive modeling and patient specific implants, today announced that they have entered into a tender offer agreement for the acquisition of all outstanding shares of Medicrea. The friendly voluntary all-cash tender offer will…
Read More4WEB Medical Announces FDA 510(k) Clearance of Stand-Alone Anterior Lumbar Interbody Fusion Device
4WEB Medical, an orthopedic device company focused on developing innovative implants with an Advanced Structural Design that utilizes its proprietary Truss Implant Technology™, announced today that the company has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its Stand-Alone Anterior Lumbar Interbody Fusion Device (ASTS-SA). The new design allows fixation screws to…
Read MoreGTX medical Granted FDA Breakthrough Device Designation for Go-2 Targeted Epidural Spinal Stimulation (TESS) System
GTX medical (GTX), today announced that the US Food and Drug Administration (FDA) has granted Breakthrough Device Designation for its implantable Go-2 system which was designed to promote the recovery of leg motor functions and neurological control in adults with spinal cord injuries (SCI) and paralysis. The Go-2 System provides Targeted Epidural Spinal Stimulation (TESS)…
Read MoreCentinel Spine Announces FDA Approval for the Manufacturing Transfer of prodisc Technology
Centinel Spine, LLC, the largest privately-held spine company focused on anterior column reconstruction, today announced FDA approval for the manufacturing transfer of both the prodisc® C Cervical Total Disc Replacement and prodisc® L Lumbar Total Disc Replacement systems to new strategic vendors. The FDA approval for manufacturing transfer is a critical milestone for Centinel Spine as it allows the…
Read MoreSmith & Nephew launches new JOURNEY II Unicompartmental Knee System
Smith+Nephew, the global medical technology business, today announces the launch of its new JOURNEY II Unicompartmental Knee (UK) System. Built on the heritage of one of the most clinically successful partial knees,1,2 and paired with proprietary OXINIUM™ Technology, JOURNEY II UK is designed to help patients rediscover their normal life. JOURNEY II UK provides a highly personalized…
Read MoreZebra Medical Vision Secures its 5th FDA Clearance, Making Its Vertebral Compression Fractures AI Solution Available in the U.S.
Zebra Medical Vision, the deep-learning medical imaging analytics company, announces today its fifth FDA 510(k) clearance for its Vertebral Compression Fractures (VCF) product. The company’s latest AI solution automatically identifies findings suggestive of compression fractures, enabling clinicians to place patients that are at risk of osteoporosis in treatment pathways to prevent potentially life-changing fractures. The…
Read MoreFDA grants Carmell Therapeutics Expedited Review for CT-101, Bone Healing Accelerant
Carmell Therapeutics, a pioneer in the development and commercialization of innovative Plasma-based Bioactive Materials (PBMs) to accelerate bone and soft tissue healing, announced that the U.S. Food and Drug Administration (FDA) last week has granted Fast Track designation for the Company’s first product, a Bone Healing Accelerant (BHA). The Fast Track designation provides the company benefits in…
Read More