Surgery and Surgical Robotics
Baxter to Expand Advanced Surgery Portfolio With Acquisition of Seprafilm Adhesion Barrier
Baxter International Inc. (NYSE:BAX), a leading global medical products company, today entered into a definitive agreement to acquire Seprafilm Adhesion Barrier and related assets from Sanofi. The agreement is the latest example of Baxter’s continued focus on acquiring products and technologies that have a strong strategic fit with the company’s leading portfolio across the hospital, including in…
Read MorePENTAX Medical Launches DEC HD Duodenoscope in the United States
PENTAX Medical, a healthcare industry leader in diagnostic and therapeutic endoscopy, has announced the United States launch of its DEC™ HD Duodenoscope, an advanced, high-definition duodenoscope that features multiple disposable components, including the sterile distal cap and elevator lever, for unit reprocessing. The DEC HD Duodenoscope is designed to provide physicians a solution aligned with recent…
Read MoreData Show Zilver PTX Leads to Fewer Complications and Shorter Hospital Stays Than Traditional Bypass Surgery
At this year’s VEITHsymposium, Dr. Marc Bosiers presented data that show that patient treatment with the Zilver PTX stent has several benefits when compared to traditional bypass surgery. The data, which were gathered from a randomized controlled trial, show that treatment with Zilver PTX results in fewer complications and shorter hospital stays for patients with…
Read MoreAvatera Robot Cleared in Europe for Minimally Invasive Laparoscopies
Avateramedical GmbH, an innovative German medical technology company, today announced the successful completion of the CE conformity assessment procedure for its avatera® system[1] for robot-assisted, minimally invasive surgery. All four components of the system – the surgical robot and control unit, the instruments, the endoscopes and the sterile components – can now carry the CE mark. This…
Read MoreCleveland Clinic First in the World to Perform Robotic Single-Port Kidney Transplant
Cleveland Clinic is the first hospital in the world to successfully perform a robotic single-port kidney transplant, which enables all surgical instruments and the donor kidney to be placed through one small abdominal incision. The Glickman Urological & Kidney Institute surgical team included Jihad Kaouk, M.D., director of the Center for Robotic and Image Guided…
Read MoreEDAP to Introduce New Endo-UP Endourology Platform During the 2019 World Congress of Endourology
EDAP TMS SA, the global leader in therapeutic ultrasound, announced today the introduction of a new concept of Endourology Platform during the WCE congress, which is being held in Abu Dhabi, October 29 – November 2, 2019. Endo-UP® is a new conceptual product designed for the management of urinary stones. Responding to the rapidly evolving…
Read MoreFirst-of-its-Kind XACT Robotic System Cleared by FDA for Percutaneous Interventional Procedures
XACT Robotics™ Ltd. today announced that its first robotic system was cleared to market in the U.S. for use during computed tomography (CT) guided percutaneous interventional procedures. XACT’s technology is the first hands-free robotic system combining image-based planning and navigation with insertion and steering of various instruments to a desired target across an array of clinical…
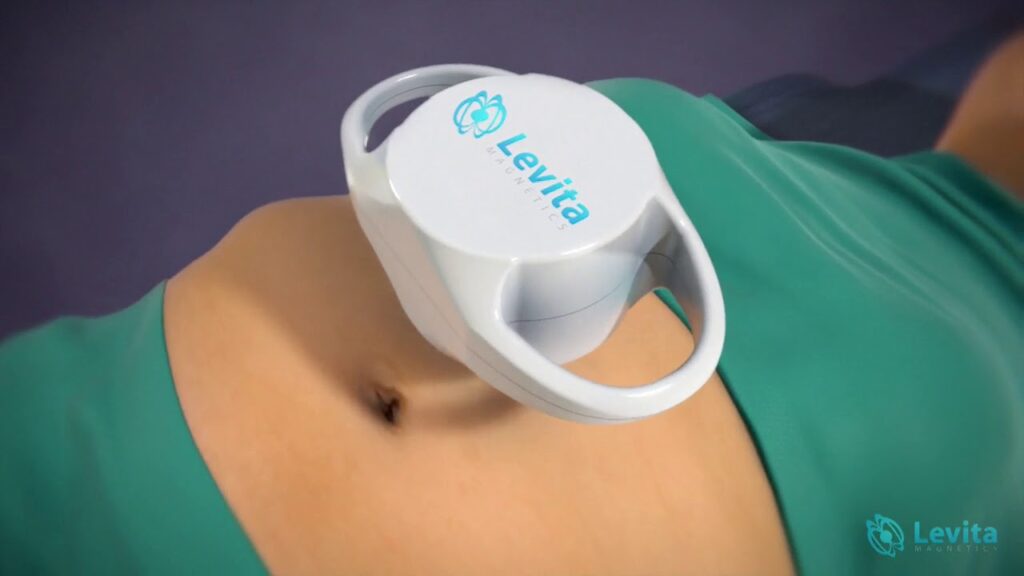
Read MoreLevita Magnetics Announces 1,000th Magnetic Surgery Procedure
Levita Magnetics, a company dedicated to improving surgical outcomes through Magnetic Surgery®, today announced that the 1,000th procedure using Magnetic Surgery has been performed. The platform represents one of the key surgical advancements in the last decade. Indicated to grasp and retract organs in certain surgical procedures, including bariatric surgery, gallbladder removal, and prostate removal,…
Read MoreNeuronetics® and Success TMS Partner to Increase Patient Access to Leading Depression Treatment, NeuroStar® Advanced Therapy
Neuronetics, Inc., a commercial stage medical technology company focused on designing, developing and marketing products that improve the quality of life for patients who suffer from psychiatric disorders, today announced a partnership with Success TMS, a healthcare provider specializing in transcranial magnetic stimulation (TMS), a non-drug, non-invasive treatment for adult patients with Major Depressive Disorder…
Read MoreU.S. FDA Clears GI Scientific’s ScopeSeal®, the Only Single-Use Disposable Device Indicated to Significantly Reduce Duodenoscope Contamination
GI Scientific, LLC, a developer of transformative innovations for gastrointestinal disease, announced that the U.S. Food and Drug Administration (FDA) cleared its ScopeSeal® Duodenoscope Protective Device, the first Endoscopic Shield® for protecting the distal end of a duodenoscope from contamination during ERCP procedures. ScopeSeal® is a single-use disposable infection control device that preserves duodenoscope optics and other…
Read More