Surgery and Surgical Robotics
Intuitive Surgical Submits New Robotic-Assisted Platform to FDA for Obtaining Lung Biopsies
Intuitive Surgical, Inc., the pioneer and a global technology leader in robotic-assisted, minimally invasive surgery, today announced it has submitted a premarket notification to the U.S. Food and Drug Administration (FDA) for the company’s new flexible robotic-assisted, catheter-based platform, designed to navigate through very small lung airways to reach peripheral nodules for biopsies. Lung cancer is the…
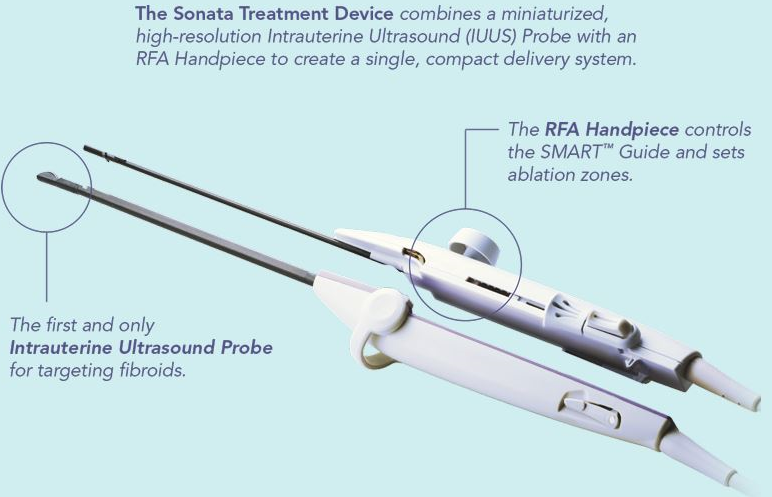
Read MoreUltrasound-Guided Transcervical Fibroid Ablation System FDA Cleared
Gynesonics, a women’s healthcare company focused on the development of minimally-invasive solutions for symptomatic uterine fibroids, today announced that it has received 510(k) clearance from the FDA to market its Sonata® Sonography-Guided Transcervical Fibroid Ablation (Sonata) System. The Sonata System combines a breakthrough integrated technology which is the first and only intrauterine ultrasound system with…
Read More7D Surgical’s Cranial Machine-Vision Guided Surgical System FDA Approved
7D Surgical announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for its Cranial Module. This achievement is a crucial step in the commercial launch of 7D Surgical’s innovative Machine-vision Image Guided Surgery (MvIGS) system for cranial surgery throughout the United States. The 7D Surgical System utilizes completely…
Read MoreImplantable Surgical Mesh may cause Autoimmune Disorders
Surgical mesh implants, often used for hernia or gynecological repair, may be the reason so many patients report symptoms of an autoimmune disorder, according to a University of Alberta rheumatologist. ‘In my practice, I studied 40 patients who had mesh implants and found that almost all of them had symptoms such as chronic fatigue, cognitive…
Read MoreNovel Intracranial Fluid Management System Receives 510(k)
IRRAS AB, a commercial-stage medical technology company focused on developing and commercializing transformative treatments to manage intracranial fluids, announced that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) for IRRAflow, its integrated, closed medical device system that enables controlled cerebrospinal fluid (CSF) management as well as accurate, continuous monitoring of intracranial pressure…
Read MoreDigital Surgery’s AI Platform Will Guide Surgical Teams Through Complex Procedures
Digital Surgery, a health tech company shaping the future of surgery through the convergence of surgical expertise and technology, today announced it has developed and successfully demonstrated the world’s first real-time, dynamic artificial intelligence (AI) system designed for the operating room (OR). The company is building the data to power the future of surgery through…
Read MoreOnkos Surgical and Insight Medical Systems Team Up On an Augmented Reality Surgical System
Insight Medical Systems has teamed up with Onkos Surgical, Inc. to explore opportunities to apply Insight Medical’s ARVIS™ (Augmented Reality Visualization and Information System) in musculoskeletal oncology. The companies are working on a pilot project to assess the technology for use in tumor surgery. ARVIS™, currently under development, has tracking and visualization capabilities which allow precise and efficient…
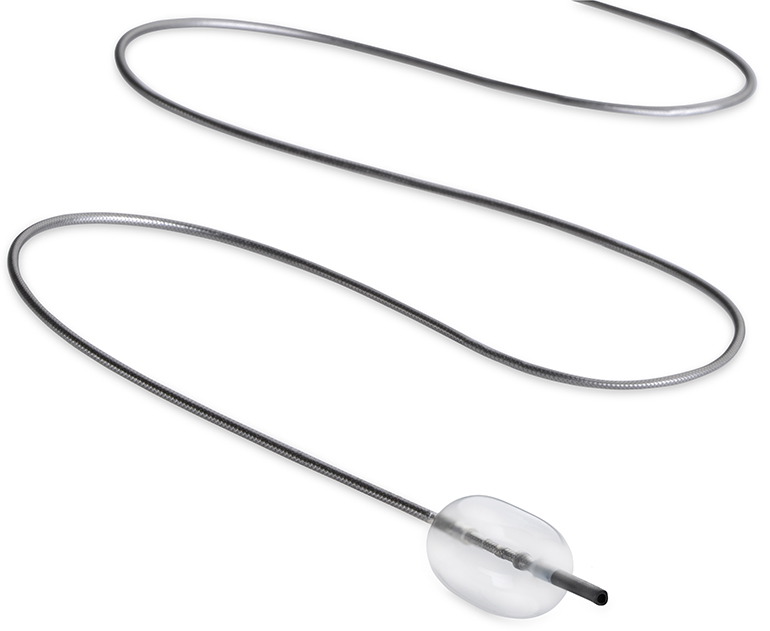
Read MoreFDA Clearance for Embolx’s Next Gen Sniper Balloon Occlusion Microcatheter
Embolx, Inc., a medical device company developing microcatheters for arterial embolization procedures, today announced that the company has received 510(k) clearance from the United States Food and Drug Administration (FDA) for its next generation family of Sniper® Balloon Occlusion Microcatheters, an innovative system for pressure-directed arterial embolization therapy. Now commercially available in the United States,…
Read MoreBrain Stimulation May Reduce Desire to Commit Sexual Assault
Stimulating the prefrontal cortex, the part of the brain responsible for controlling complex ideas and behaviors, can reduce a person’s intention to commit a violent act by more than 50 percent, according to research published in the Journal of Neuroscience from the University of Pennsylvania and Nanyang Technological University. What’s more, using such a minimally invasive technique, called transcranial direct-current…
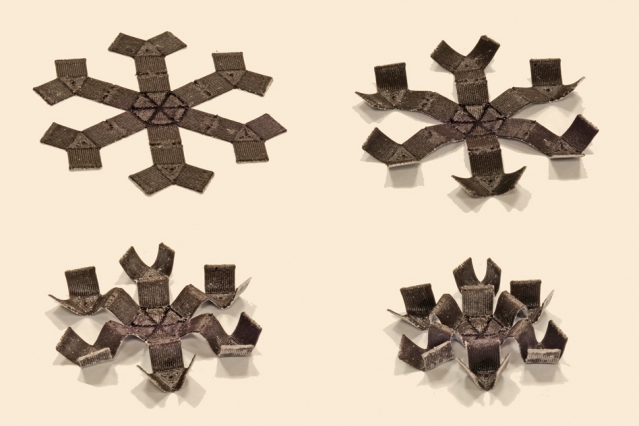
Read MoreMIT’s 3D Printed Walking, Drug-Transporting Magnets
MIT engineers have created soft, 3-D-printed structures whose movements can be controlled with a wave of a magnet, much like marionettes without the strings. The menagerie of structures that can be magnetically manipulated includes a smooth ring that wrinkles up, a long tube that squeezes shut, a sheet that folds itself, and a spider-like “grabber”…
Read More