Surgery and Surgical Robotics
3NT Medical Announces FDA Clearance for Colibri Endoscopy System
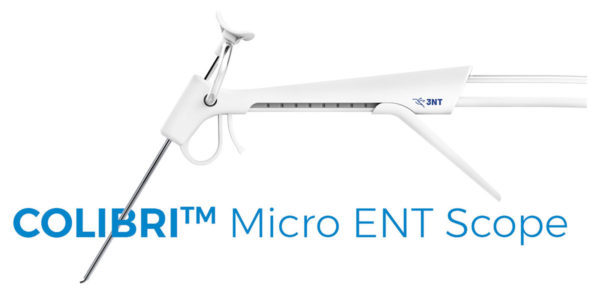
3NT Medical, a privately-held corporation dedicated to developing single-use specialized endoscopes for the diagnosis and treatment of ear, nose and throat (ENT) disorders, announced today the FDA 510(k) clearance for the Colibri™ Micro ENT Scope, the world’s first single-use endoscope specifically designed for Otology. The Colibri™ endoscopy system incorporates a lightweight ergonomic handpiece, a 2.2mm…
Read MoreGynesonics Receives FDA Clearance to Market Next Generation Sonata System 2.1
Gynesonics, a women’s healthcare company focused on the development of minimally-invasive solutions for symptomatic uterine fibroids, announced today that it has received 510(k) clearance from the U.S. Food and Drug Administration (FDA) to market its next generation Sonata® System 2.1 for Transcervical Fibroid Ablation (TFA). The Sonata technology platform integrates the first and only intrauterine…
Read MoreSMART Medical Systems Receives FDA Clearance for Its G-EYE Colonoscope
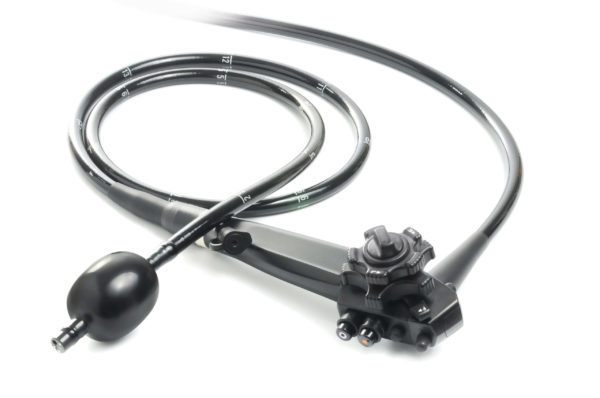
SMART Medical Systems Ltd., a developer and manufacturer of innovative endoscopy products, announced that the FDA issued 510(k) clearance for its flagship product, the G-EYE® Colonoscope. The G-EYE® colonoscope is a standard colonoscope which SMART remanufactures by installing its proprietary G-EYE® balloon on the distal bending section of the colonoscope. During colonoscopy, the G-EYE® colonoscope is inserted in the…
Read MoreNevro Announces CE Mark Approval of Senza Omnia Spinal Cord Stimulation System to Treat Chronic Pain
Nevro Corp., a global medical device company that is providing innovative, evidence-based solutions for the treatment of chronic pain, today announced it has received CE mark approval for the Senza® Omnia™ Spinal Cord Stimulation (SCS) system. “Following a successful launch in the United States late last year, we are excited to now have approval for the Senza Omnia…
Read MoreAmbu Launches New Single-Use Cystoscope for Improved Workflow and Enhanced Productivity Benefits
Ambu Inc., a rapidly growing medical device maker and pioneer of sterile, single-use endoscopes, introduced today a new single-use cystoscope. The Ambu® aScope™ 4 Cysto will give urologists immediate access to a single-use cystoscope that can be used for procedures such as bladder cancer surveillance, stent removal and other common cystoscopy procedures. Built on more…
Read MoreFDA Clears New Mechanical Thrombectomy Platform
Control Medical Technology announced the FDA cleared the Aspire MAX 7 – 11F Mechanical Thrombectomy platform to remove blood clots from peripheral vessels. Blood clot removal (thrombectomy) is a common procedure. Coronary thrombectomy is associated with acute myocardial infarction (AMI), neurovascular thrombectomy is associated with acute ischemic stroke, and peripheral thrombectomy is associated with peripheral…
Read MoreAllurion Technologies Announces Submission of US Premarket Approval Application for its Flagship Elipse Gastric Balloon
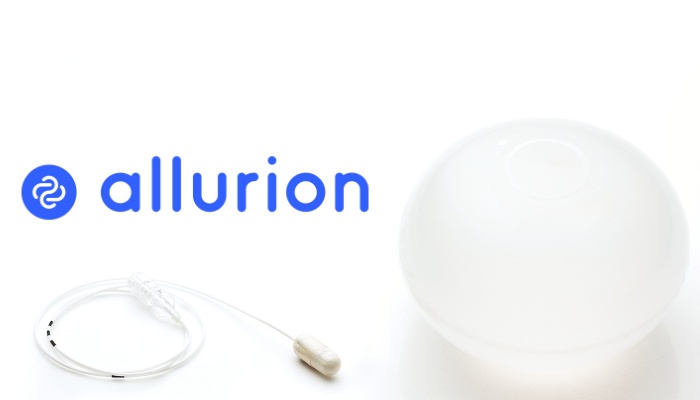
Allurion Technologies, an established leader in the development of innovative, proven, and trusted weight loss experiences, today announced the Premarket Approval (PMA) submission of its flagship Elipse gastric balloon. The company had previously announced the successful completion of its landmark FDA pivotal study and the hiring of key personnel who will be leading the US market launch. These milestones…
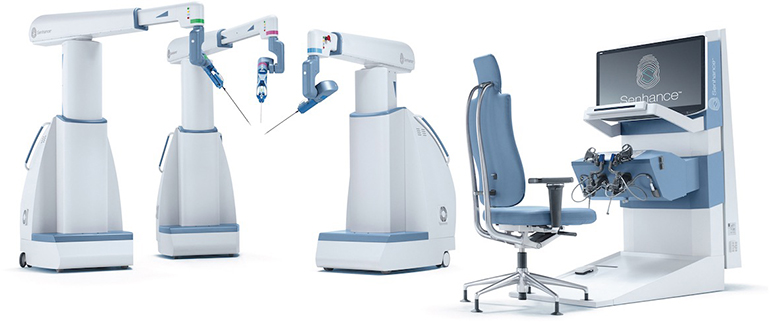
Read MoreTransEnterix Receives FDA Clearance for First Machine Vision System in Robotic Surgery
TransEnterix, Inc., a medical device company that is digitizing the interface between the surgeon and the patient to improve minimally invasive surgery, today announced the Company received 510(k) clearance for the Intelligent Surgical Unit (ISUTM) that enables machine vision capabilities on the SenhanceⓇ Surgical System. “We are pleased to have received this important clearance earlier than…
Read MoreEndologix Announces FDA Approval of AFX Endovascular AAA System
Endologix, Inc. (Nasdaq: ELGX), developer and marketer of minimally invasive treatments for aortic disorders, announced today that it has received U.S. Food and Drug Administration (FDA) approval for its next generation product, the AFX™ Endovascular AAA System, for the treatment of abdominal aortic aneurysms (AAA). Endologix is introducing AFX at the Annual Meeting of the Society…
Read MoreAerin Medical Announces FDA Clearance and U.S. Launch of Innovative Nonsurgical Procedure for Chronic Rhinitis
Aerin Medical Inc., a company focused on minimally invasive solutions for chronic nasal conditions, today announced U.S. Food and Drug Administration (FDA) clearance and U.S. launch of the company’s second product, the RhinAer™ Stylus, an innovative device for nonsurgical treatment of chronic rhinitis. More than 30 million Americans suffer from nonallergic rhinitis.1 Patients with the condition…
Read More