Medical device and MedTech insights, news, tips and more
FDA Warns Against Using Surgical Robots in Cancer Surgeries
March 4, 2019
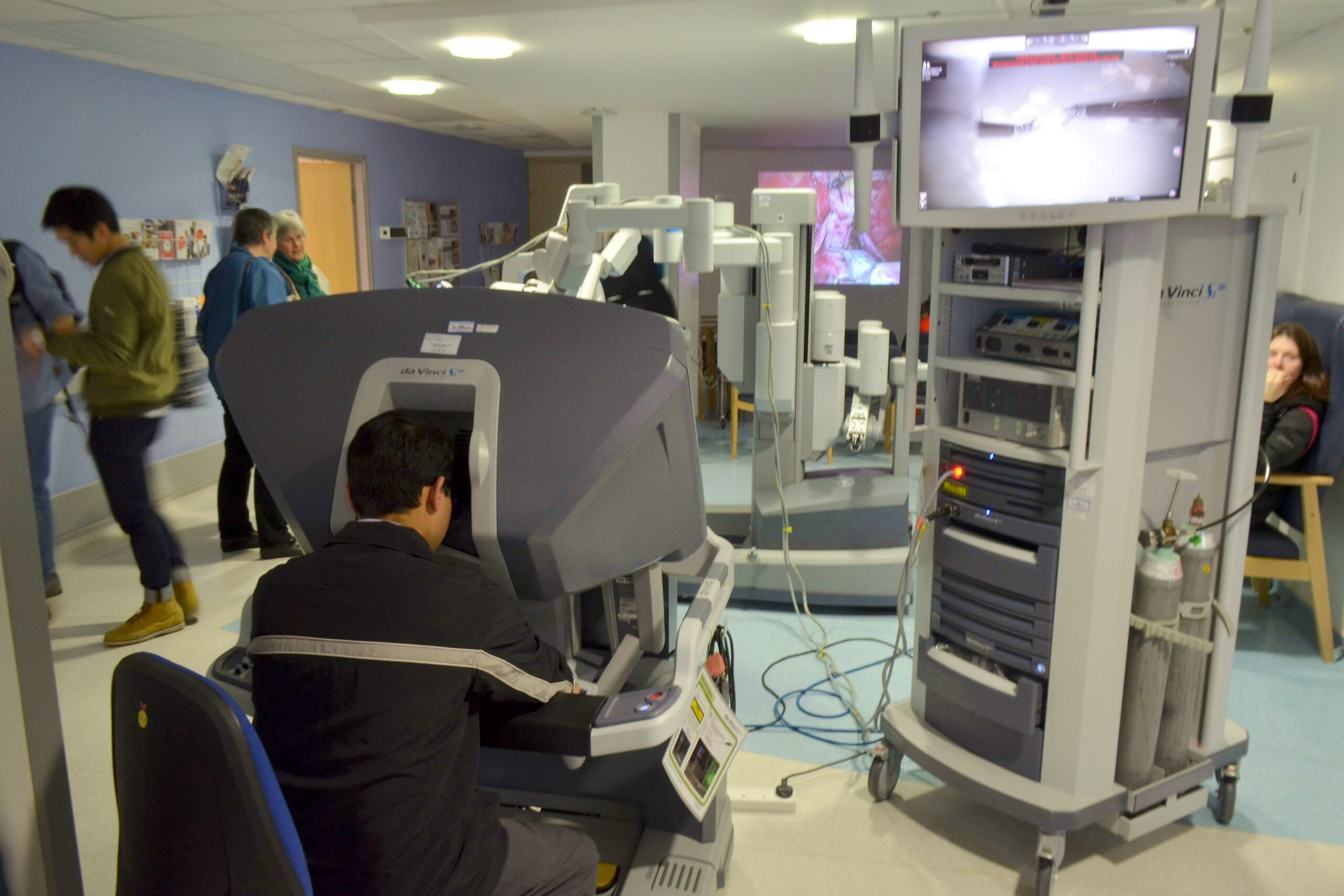
The Food and Drug Administration on Thursday warned against the use of robotically assisted devices for mastectomies and other cancer surgeries, asserting the products may pose safety risks and result in poor outcomes for patients.
The agency said it decided to issue its warning after reviewing studies suggesting that robotically assisted devices were being used to perform cancer procedures for which there is limited data on their safety and effectiveness. The FDA has not authorized the devices for mastectomy or for the treatment or prevention of cancer.
One recent study reported that use of the devices in radical hysterectomies in women with cervical cancer was associated with lower rates of disease-free survival and overall survival than traditional surgery.
“We want doctors and patients to be aware of the lack of evidence of safety and effectiveness for these uses so they can make better informed decisions about their cancer treatment and care,” said Dr. Terri Cornelison, assistant director for the health of women in the FDA’s Center for Devices and Radiological Health.
Use of robotically assisted surgical devices is increasing because they allow surgeons to perform procedures through smaller incisions, helping to reduce pain, blood loss, scarring, infection, and recovery time. In such surgeries, computer technology enables a surgeon to control instruments attached to mechanical arms while viewing the surgical site in three-dimensional, high-definition video.
See Full Story at the Source: FDA sounds an alarm on using robotic devices in cancer surgeries – STAT
Written by: Casey Ross, with Sharon Begley contributing
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
