Medical device and MedTech insights, news, tips and more
Heidelberg Engineering wins FDA nod for OCT Angiography Module
September 20, 2018
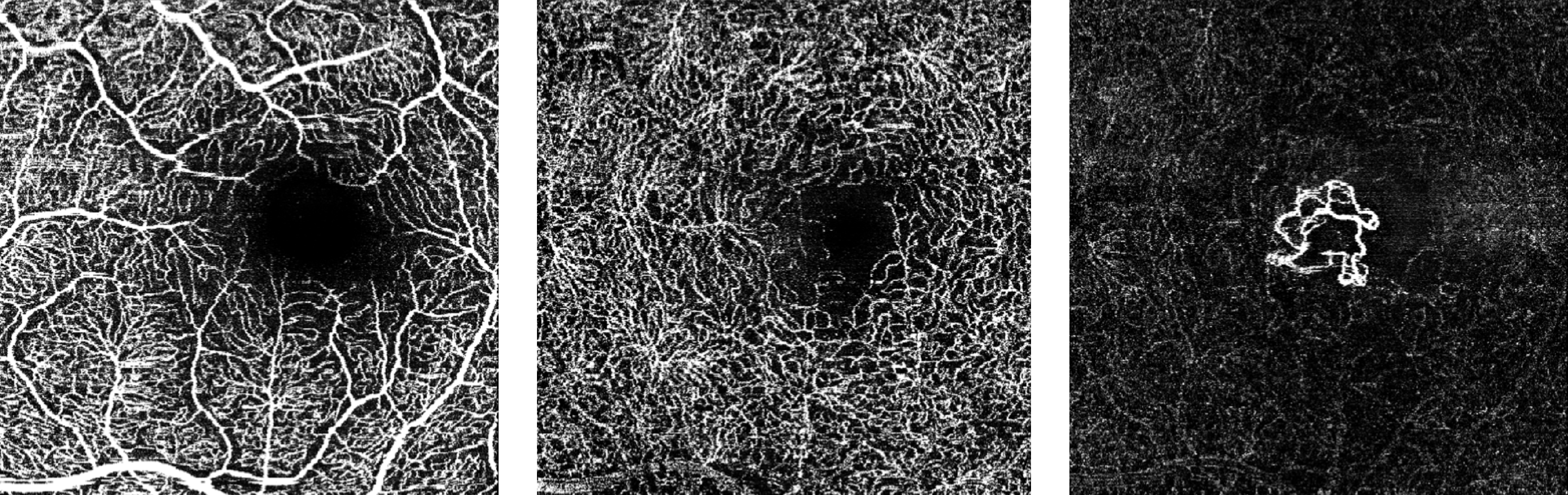
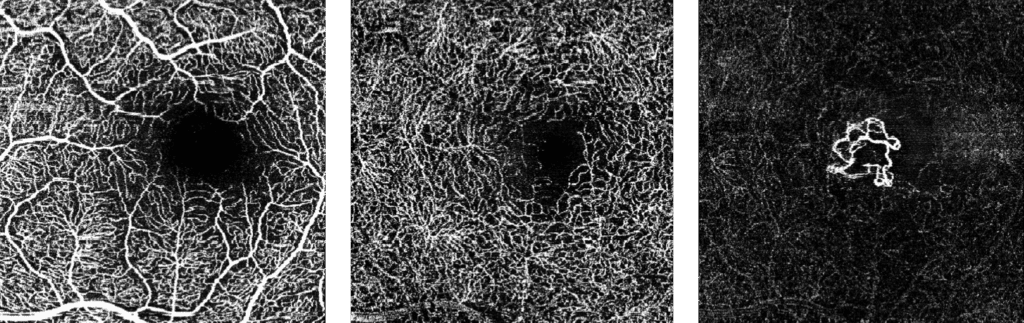
The FDA has approved Heidelberg Engineering’s optical coherence tomography angiography module (OCTA), the company said today. The module is available for new and existing Spectralis upgradeable diagnostic imaging devices used to manage diabetic retinopathy, age-related macular degeneration and other macular diseases.
OCTA is a noninvasive imaging technique that provides three-dimensional visualization of perfused ocular vasculature. Structural OCT uses light waves to take cross-section pictures of the retina, allowing the ophthalmologist to map and measure the thickness of each of the retina’s distinctive layers. Combined with dye-based angiography, the OCTA technology is designed to give clinicians a more comprehensive understanding of ocular abnormalities.
Since the original 510(k) clearance of OCT angiography, there have been significant advancements in the technology and specifically in its clinical applicability, making the validation of the technology a much more rigorous task, according to a statement from the company.
“Our international experience with the Spectralis OCTA module has confirmed the significance of our live eye tracking and dynamic angiography to acquire clinically relevant data,” said Ram Liebenthal, general manager of the Franklin, Mass. company, in the statement. “It also demonstrates our continued dedication to delivering high-resolution images, powerful clinical tools, and a multimodal diagnostic approach to aid eye care professionals in the detection and management of diverse eye conditions, all on a single platform.”
Read More at the Source: Heidelberg Engineering wins FDA nod for upgraded eye imaging technology | Medical Design and Outsourcing
By: Nancy Crotti
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.