Medical device and MedTech insights, news, tips and more
Nexus Ultrasonic Surgical Platform from Misonix FDA Cleared
June 6, 2019
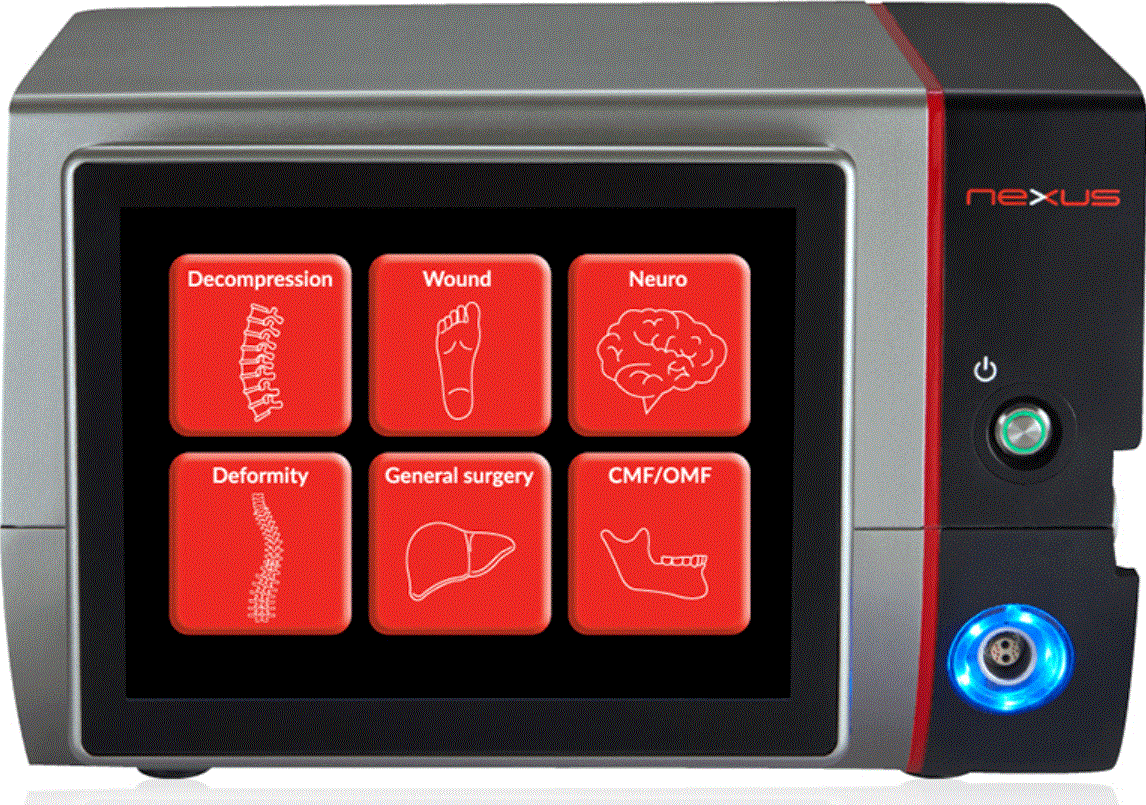
Misonix, Inc., a provider of minimally invasive therapeutic ultrasonic medical devices that enhance clinical outcomes, today announced that it received 510(k) clearance by the U.S. Food and Drug Administration (FDA) for Nexus, its revolutionary ultrasonic surgical platform. Misonix will commence the commercialization of the Nexus platform in the United States in July.
Nexus is a next-generation integrated ultrasonic surgical platform that combines all the features of Misonix’s existing solutions, including BoneScalpel, SonicOne and Sonastar, into a single fully integrated platform that will also serve to power future solutions. The Nexus platform is driven by a new proprietary digital algorithm that results in more power, efficiency and control. Nexus uniquely incorporates RF capabilities, allowing for use in general surgery procedures. The device also incorporates Smart Technology that allows for easier setup and use.
Physicians will be able to utilize Nexus’ increased power to improve tissue resection rates, in concert with its proprietary digital algorithm to perform more efficient bone removal procedures. In addition, Nexus’ ease of use will enable physicians to fully leverage Nexus’ impressive capabilities via its digital touchscreen display and smart system setup.
Stavros Vizirgianakis, President and Chief Executive Officer of Misonix, commented, “We are very pleased to have achieved this critical approval, marking a significant milestone for Misonix and the culmination of years of hard work. Nexus is a powerful, highly integrated and easy-to-use system that will benefit both healthcare providers and patients by incorporating the latest advances in ultrasonic technology allowing for increased efficiency and efficacy, and thus improved outcomes. It is truly a transformational product.
“The overwhelmingly positive feedback we have received from the surgeon community since first unveiling Nexus reinforces our confidence in its potential to serve as a significant growth engine for Misonix. Nexus is a key component of our strategy to increase our presence in the neuro, spine, ortho, wound and general surgery markets. Nexus provides us with a unique opportunity to leverage our robust consignment business model, further drive the sale of consumables competitively, and extend our ability to cross-sell into multiple physician specialties. The commercialization of Nexus will expand the utilization of ultrasonic surgical applications as we continue to strategically invest in our products to become the standard of care in operating rooms and hospital outpatient departments.”
See Full Press Release at the Source: Misonix Receives U.S. FDA Clearance for Nexus, its Revolutionary Integrated Ultrasonic Surgical Platform – Misonix
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

