Medical device and MedTech insights, news, tips and more
Northwestern Memorial Hospital Performs First US Robotic Lung Volume Reduction Surgery
January 21, 2019
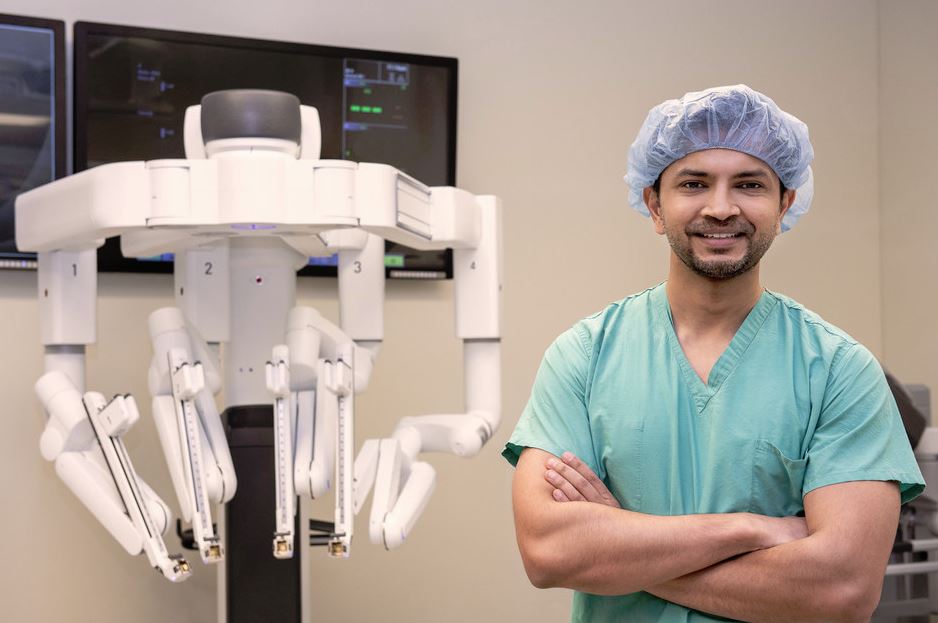
Northwestern Memorial Hospital is the first in the country to successfully complete robotic-assisted lung volume reduction surgery (LVRS) using the da Vinci Xi Surgical System® to remove diseased, emphysematous tissue within the lungs for the treatment of severe emphysema, a progressive form of chronic obstructive pulmonary disease (COPD). The robotic procedure allows the surgeon to precisely remove the diseased portions of the lungs, which may reduce pain and scarring, lower the risk of infection and shorten recovery time compared to traditional robotic lung volume reduction surgery.
“Robotic-assisted technology is revolutionizing the way patients are treated for chronic lung diseases,” said Ankit Bharat, MD, surgical director of the Lung Transplant Program & ECMO at Northwestern Memorial. Dr. Bharat and a team of highly skilled clinicians performed the first procedure late last year. “By providing this innovative surgical option to our patients, we are changing the paradigm of emphysema management by expanding the possibilities to our patients who desperately need an option to improve breathing to enhance their quality of life and improve overall health.”
During the robotic LVRS, a surgeon with specialized training in robotic surgery sits at the computer console and looks through the stereoscopic, High Definition (HD) monitor to see inside the patient, giving the surgeon a better, more detailed 3D view of the operating area than the human eye can offer. The tower, which is positioned directly over the patient during surgery, contains the robot’s four arms—three that can hold a multitude of different surgical instruments, and a fourth that holds the system’s 3D cameras. These arms are controlled by a computer that exactly replicates the movements of the operating surgeon.
Using the master controls, the surgeon maneuvers the arms of the robotic device to make three small incisions on the right side of the chest accessing the lungs and removing the diseased cysts called blebs. This lets the remaining lung tissue function better for optimal breathing. By removing the non-function lung tissue, the diaphragm, chest wall, and rib cage are able to return to a more normal shape and work more efficiently.
Unlike the traditional surgical approach that uses a larger chest incision to access the lungs, the robotic LVRS only requires three 8-millimeter incisions. The smaller incisions reduce scarring and minimize the risk for infection, while also potentially reducing the length of stay in the hospital.
“We are excited to expand the therapeutic options we can now offer to a broader range of patients who need surgical intervention and are not responding to medications or pulmonary rehabilitation,” Ravi Kalhan, MD, pulmonologist and medical director of the LVRS program at Northwestern Memorial. “This new option demonstrates our team’s determination to deliver superior care and a level of expertise not found anywhere else in the country.”
Kalhan adds not every patient is a good candidate for robotic LVRS and must fit specific criteria in order to be considered for surgery. “Patients must be diagnosed with severe COPD with limited exercise capacity after completing pulmonary rehabilitation.”
Emphysema is classified as a form of COPD caused by damage to the alveoli, the tiny air sacs in the lung where the exchange of oxygen and carbon dioxide occurs. With emphysema, damage to the alveoli results in air becoming trapped, causing them to expand and rupture. As a result of the progressive damage to alveoli, people with emphysema experience a decreased level of oxygen in the blood combined with an increased level of carbon dioxide in the blood. Currently 16 million Americans are affected by COPD according to the U.S. Centers for Disease Control and Prevention.
Source: Northwestern Memorial Hospital First in the U.S. to Perform Robotic Lung Volume Reduction Surgery
Press Release by Northwestern Memorial Hospital
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.