Medical device and MedTech insights, news, tips and more
Novarad Lands FDA Clearance for its HoloLens-augmented Preoperative System
October 30, 2018
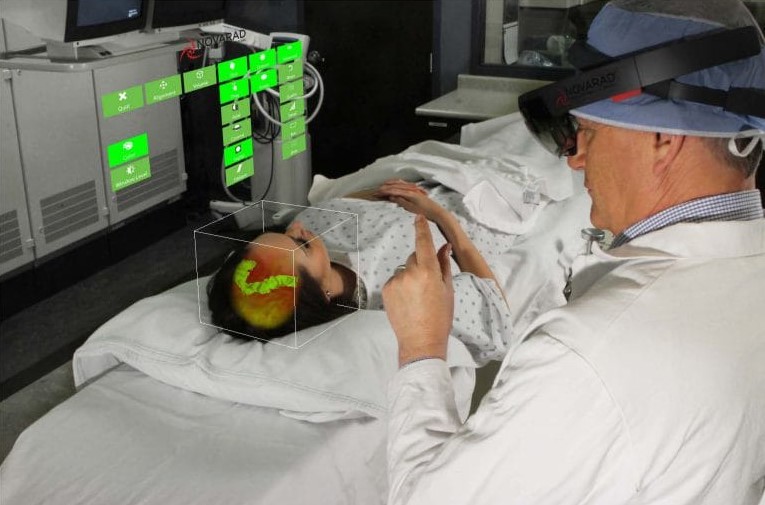
The possibilities of Microsoft’s HoloLens system have become a hot topic in the medical world in recent years. But last week Novarad’s OpenSight Augmented Reality System received FDA clearance for pre-operative surgical planning using the tech company’s augmented reality headset.
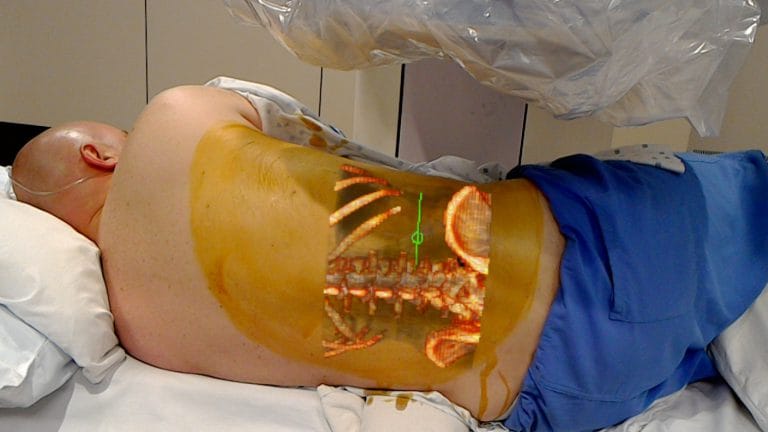
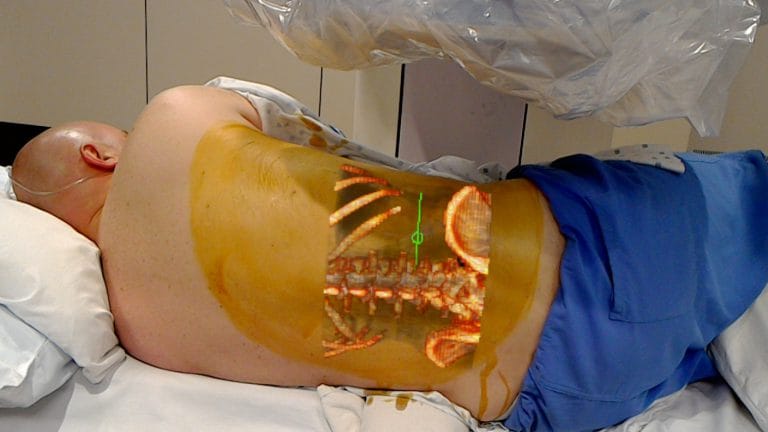
The newly FDA-cleared system uses the lens to project 2D, 3D and 4D interactive images onto a patient’s body during surgery. The system lets clinicians see both the 3D patient image from previous scans as well as the patient physically in front of them. OpenSight was designed to help surgeons plan for an operation and gives clinicians the tools to highlight specific body parts as well as structures that should be avoided during the procedure, according to the company.
The technology — which according to Novarad is the first using HoloLens to be cleared by the FDA — can be used by surgeons, radiologist and students. It also lets users interact with the imaging data and see the picture displayed in an anatomically correct way on the patient, according to the website.
Why it matters
Recently doctors and developers have been turning to augmented reality as a way to plan for surgeries, as well as provide surgeons more resources during the operation itself. Both Microsoft’s HoloLens system and Google Glass have become popular catalysts for scans and information to be relayed through during surgery.
Augmented reality technology gives practitioners the ability to see previous scans — often times with notes or highlights. In some use cases a remote physician can provide guidance and notes via the technology.
The trend
Uses cases for the HoloLens technology can be seen all over the world. In August, Alder Hey Children’s Hospital and NHS Foundation Trust announced that it would be using the augmented reality technology. The hospital said that alongside the technology, it was also developing an app to help surgeons better access information about their patients.
Additionally, in January a study published in the European Radiology Experimental outlined how surgeons at St. Mary’s Hospital in London used Microsoft HoloLens to overlay images of CT scans onto patients’ legs during reconstructive lower limb surgery.
On the record
“This is transformative technology that will unite preoperative imaging with augmented reality to improve the precision, speed and safety of medical procedures,” Dr. Wendell Gibby, Novarad CEO and co-creator of OpenSight, said in a statement. “This internal visualization can now be achieved without the surgeon ever making an incision, improving outcomes in a world of more precise medicine.”
Read More at the Source: Novarad lands FDA clearance for its HoloLens-augmented preoperative system | MobiHealthNews
By: Laura Lovett
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.