Medical device and MedTech insights, news, tips and more
Serenno Medical Introduces Novel Device for Continuous Automatic Monitoring of Kidney Function of Hospitalized Patients
February 5, 2020
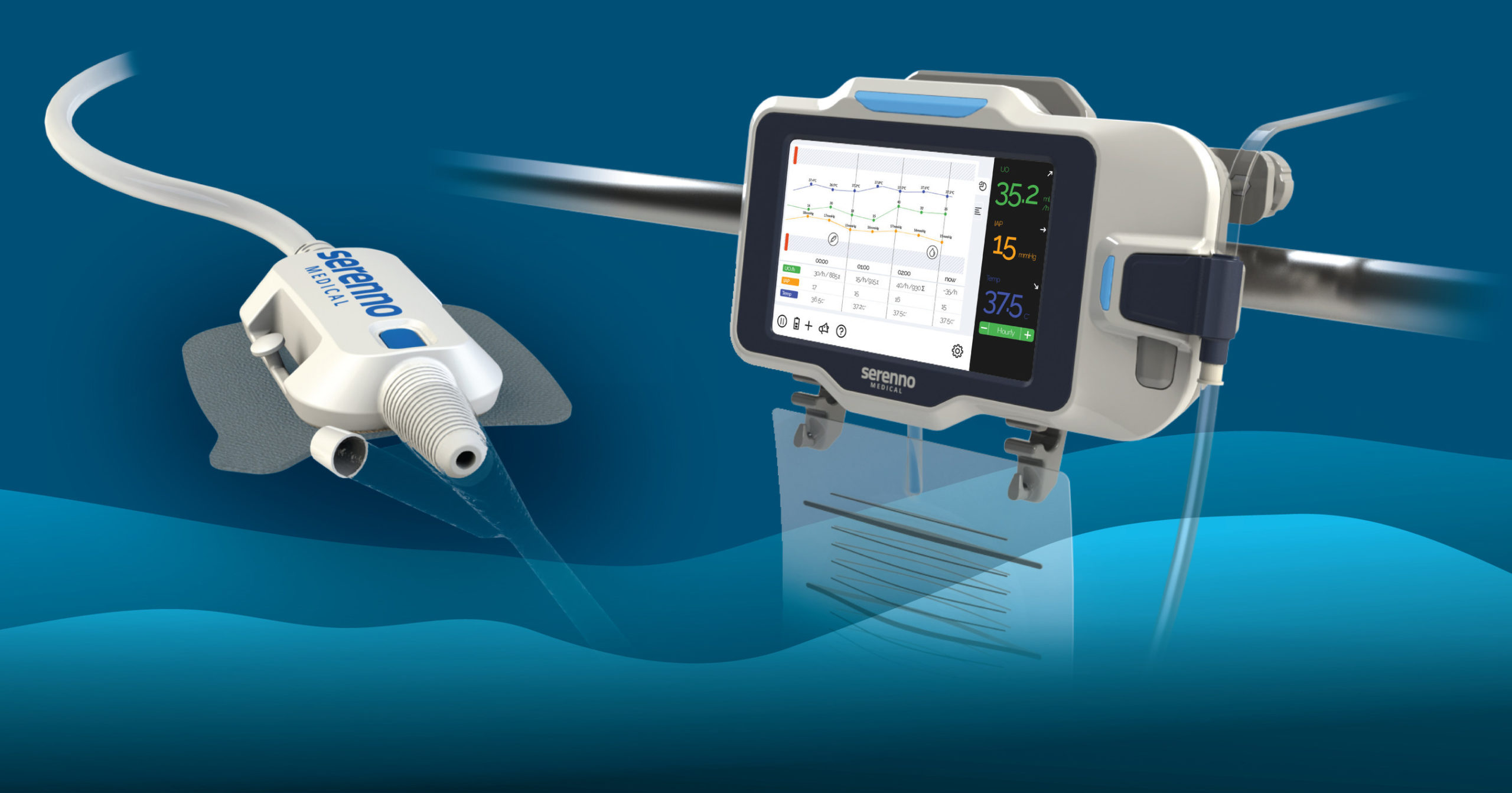
Serenno Medical, developer of medical devices for patient monitoring in a hospital setting, reveals Sentinel™, a novel, robust device for automatic monitoring and detection of kidney damage in hospitalized patients. The Sentinel device is a continuous urine output monitor that measures urine flow rate and volume in real time.
Continuous kidney function assessment allows the early detection of Acute Kidney Injury (AKI), a common condition in hospitalized patients that significantly increases risk of mortality during and after hospitalization. Accurate measurement of urine output (UO)
is clinically accepted as the best method for monitoring kidney function. However, UO is currently monitored intermittently and manually by ICU nurses, therefore acute changes in urine flow are difficult to detect. Thus, kidney injury is often detected relatively late, sometimes after it is impossible to prevent further progression.
Sentinel offers a simple and cost-effective solution for the precise, continuous measurement of urine flow rate in real time. The system promotes early detection of kidney injury, while there is still time to intervene and prevent further damage. It aims to automatically and accurately detect small changes in kidney function, taking the workload off the nursing staff while delivering actionable data. The device works in synergy with existing hospital equipment (any catheter and bag) and requires a short and simple, non-invasive installation. It fits the complicated intensive care unit (ICU) and operating room environments and is fully functional in any patient condition or hospital environment.
Prof. Manu Malbrain, MD, PhD, ICU Director, University Hospital Brussels, Co-founder and Former-President WSACS and Co-founder and President of the International Fluid Academy, said, “Urine output measurement is a key parameter to assess kidney function. However, correct urine output determination is often difficult at the bedside and is usually only performed intermittently and manually. Moreover, the kidneys are often the first organ to stop functioning properly, therefore continuous urine output measurement would be a valuable tool in the management of critically ill, unstable patients.”
Serenno Medical recently completed its first multicenter clinical trial with the Sentinel system, showing high reliability and accuracy in a wide range of environments and patient conditions, including low and high urine flow rates, regardless of patient position, during surgical procedures, and including continuous monitoring of patients in a mobile hospital bed. The clinical trial, conducted at three ICU units in Sheba and Rabin Medical centers in Israel, included more than 40 patients and over 1,300 hours of monitoring. Results show over 96% accuracy and reliability.
“Serenno has developed a unique product that will bring tremendous value to patients hospitalized in ICU units along with a simple digital solution that will bring great economic value to hospitals,” stated Shimon Eckhouse, PhD, Chairman of Serenno Medical. “Serenno’s successful clinical trial is a great testament to the quality and performance of the Company’s product.”
“The successful results of our first clinical trial will allow the company to move forward to manufacturing the device for commercial usage and enable us to bring this best-in-class product to the global medical markets,” said Tomer Lark, Co-Founder and CEO of Serenno Medical. “We expect to receive FDA clearance for the system during 2020 and believe that our patent pending device has the potential to become the standard-of-care in the OR and ICU, benefiting millions of patients around the world.”
See Full Press Release: Serenno Medical Introduces Novel Device for Continuous Automatic Monitoring of Kidney Function of Hospitalized Patients
Written by: Serenno Medical

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

