Medical device and MedTech insights, news, tips and more
TAP At-Home Blood Collection System Now FDA Cleared
January 3, 2020
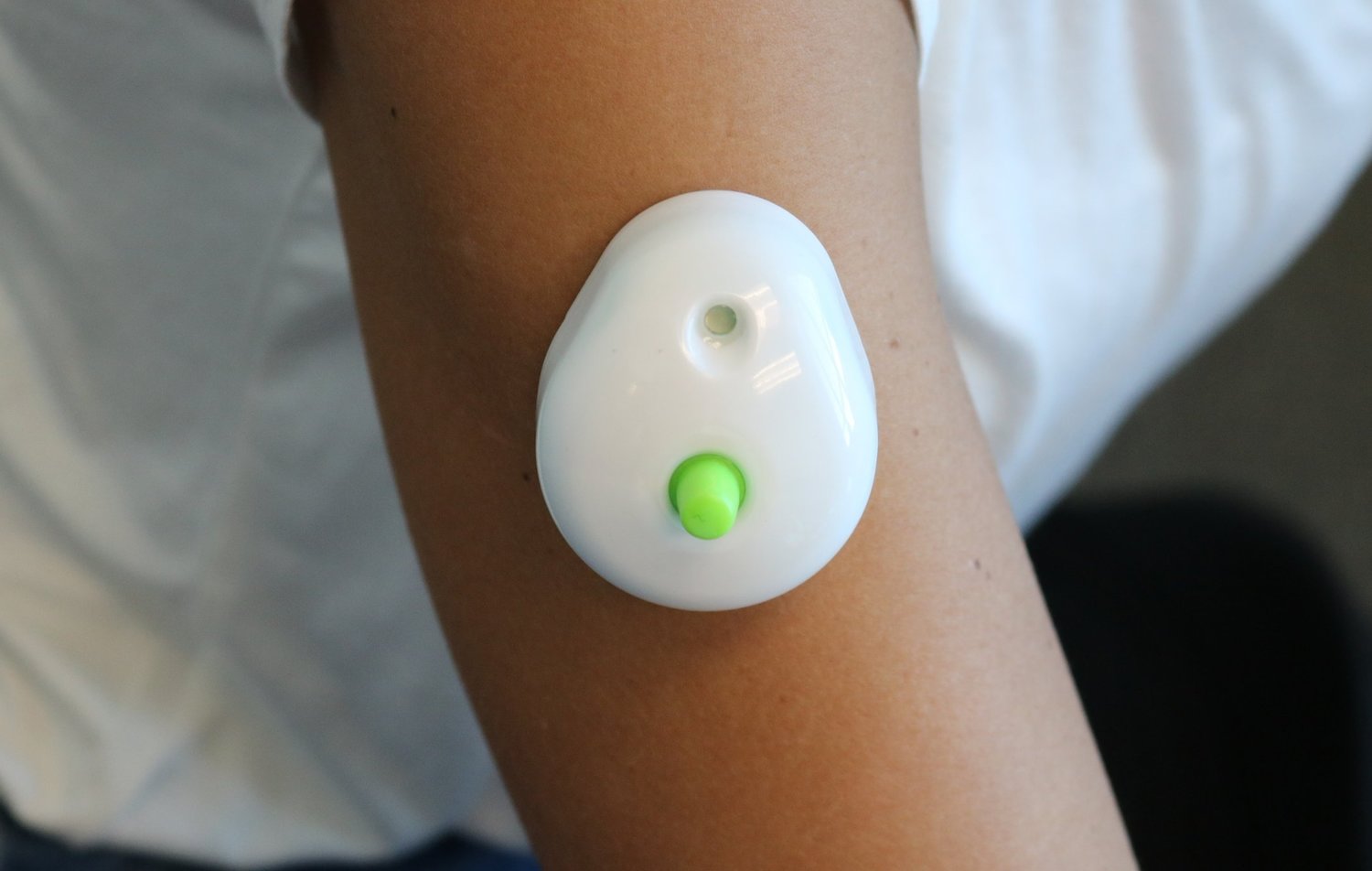
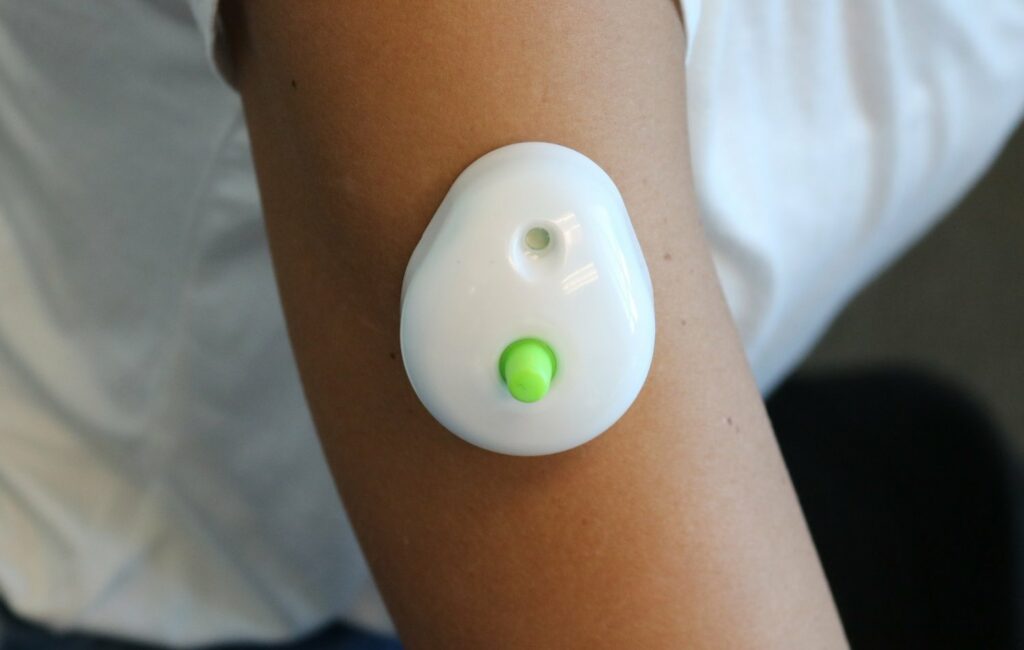
Seventh Sense Biosystems, Inc. (7SBio) announced today that the company has received U.S. Food and Drug Administration (FDA) 510(k) authorization to extend its existing clearance to include blood collection by laypersons. Regulators are also allowing the device to be used ‘at-home’ for wellness testing.
“We’re very excited about this clearance since it represents an unprecedented landmark event in healthcare and, more importantly, has the potential to significantly improve patient outcomes,” said Rick Bente, CEO of 7SBio. “We believe that TAP will transform blood collection from an inconvenient, stressful, and painful experience to one where users can perform nearly painless blood collection themselves anywhere. The company aims to make monitoring much easier for both healthcare professionals and patients without sacrificing the accuracy of standard lab testing.”
Easy to Use and No More Large Needles to Fear
For decades, only healthcare professionals have been allowed to collect blood for testing. With TAP, people can now control the process and experience virtually painless and convenient at-home blood collection for wellness testing. TAP is placed on the upper arm and blood collection starts with a simple press of a button – the process typically takes 2-3 minutes. After collection, TAP can easily be sent back to standard labs for wellness testing.
An Important Step Forward
7SBio’s vision is to lead the “self-collection” movement – enabling consumer-friendly blood collection to occur wherever and whenever needed. “With all the innovation occurring in healthcare, blood collection has lagged far behind and is still a primitive and difficult process for so many people,” said Dr. Robert Langer, 7SBio Founder, Board Member and one of 12 Institute Professors at MIT. “I, and so many people I know, are excited about the potential for this device to help people understand their health status as early as possible as well as to save them time and reduce pain.”
About Seventh Sense
Seventh Sense Biosystems has designed and developed TAP, the world’s first push-button blood collection device. It makes the process simple, convenient, and painless. Approximately $50 billion is spent annually on diagnostics and consumers are demanding more from the healthcare system. The company aims to create a new standard for blood collection that increases patient compliance with testing orders, leading to faster diagnoses and better outcomes. The company’s recent clearance may be found on the FDA website. 7SBio is funded by aMoon Fund, Flagship Pioneering, Newpath Partners, and Polaris Partners, as well as LabCorp ($LH), Miraca ($MRCHF), and Novartis ($NVS). The Company was founded by Flagship Pioneering along with academics from MIT, executives from the pharmaceutical and diagnostic industries, and a former general counsel from the FDA. For more information: www.7sbio.com.
See Full Press Release: Seventh Sense Biosystems Unlocks Market for Consumer Blood Collection Through Layperson Clearance
Written by: Seventh Sense Biosystems

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.