Medical device and MedTech insights, news, tips and more
TSolution Announces FDA Approval for a Robot Capable of Performing a Total Knee Replacement
October 14, 2019
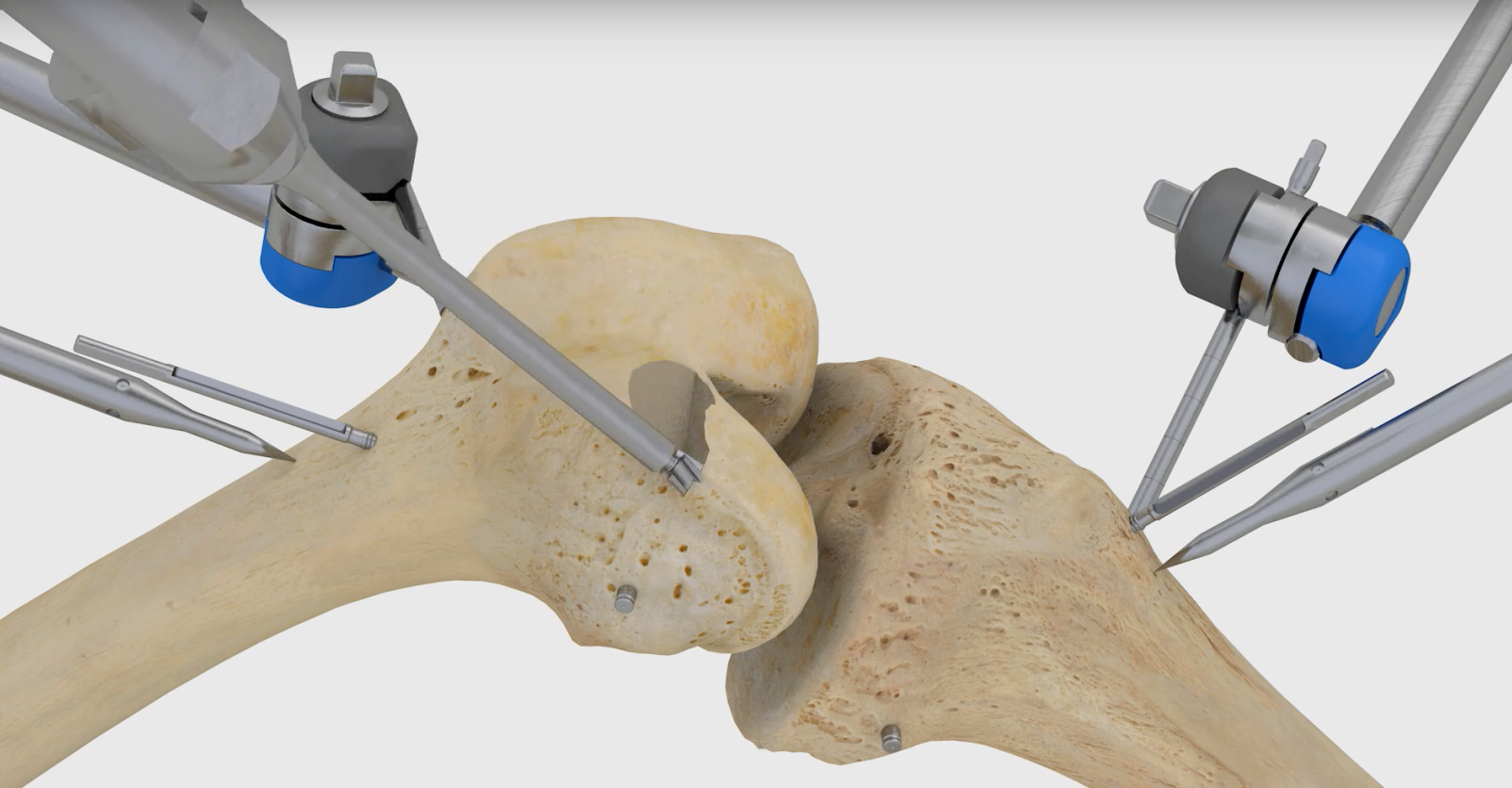
Think Surgical Inc. this week announced that the U.S. Food and Drug Administration has granted clearance for it to market the TSolution One system for total knee replacement in the U.S. The robot already has CE Marking, and surgeons have used it in more than 550 procedures in the Asia-Pacific and European markets.
“We are thrilled to expand the use of this state-of-the-art technology, which is truly transforming orthopedic surgery,” stated John Hahn, CEO and president of Think Surgical. “FDA clearance of TSolution One furthers our commitment to improving the lives of patients suffering from severe osteoarthritis by expanding the global commercialization of our active robot for TKA [total knee arthroplasty].”
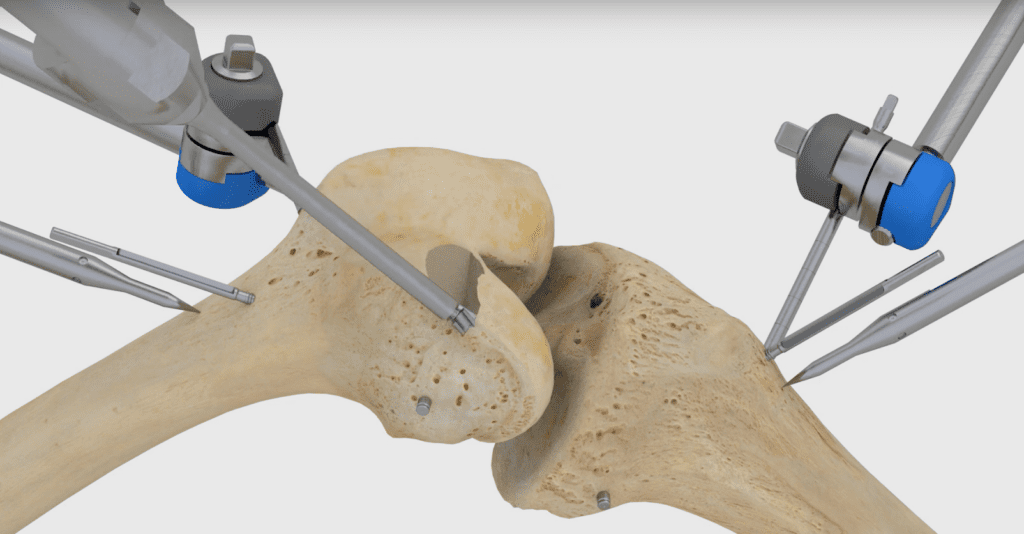
In March, Think Surgical raised $134 million in funding. It joins Stryker Corp.‘s Mako system, Smith & Nephew PLC’s Navio, and several other companies in the competitive orthopedic market.
TSolution One technology and trials
The TSolution One total knee application includes CT-based, 3D pre-surgical planning software that enables a surgeon to prepare, in a virtual environment, the patient’s unique joint-replacement plan using a number of implant options.
During total knee replacement surgery, the surgeon implements the patient’s preplanned procedure using the robot, which prepares the joint according to the surgeon’s plan for precise placement of implants.
Five surgeons participated in the clinical trial, which confirmed the safety and efficacy of the TSolution One in comparison with traditional knee replacement using manual surgical instrumentation. Study enrollment was completed in December 2018 and included 115 patients.
The surgeons who participated in the study included Bernard Stulberg in Cleveland; Yair David Kissin in Hackensack, N.J.; Stefan Kreuzer in Houston; Ralph Liebelt in Durham, N.C.; and William Long in New York.
“What will be particularly appealing to surgeons is the open implant library offered by this active robotic system, which can achieve an optimal personalized surgical plan for each patient,” said Dr. Stulberg, an orthopedic surgeon and principal investigator in the U.S. clinical study for TSolution One. “This innovative system will revolutionize the treatment of end-stage knee arthritis by coupling an individualized plan with precise bone cutting technology.”
See Full Article: TSolution One robot for total knee replacement receives FDA approval
Written by: The Robot Report Staff

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
