Medical device and MedTech insights, news, tips and more
Ultrasound-Guided Transcervical Fibroid Ablation System FDA Cleared
August 24, 2018
Gynesonics, a women’s healthcare company focused on the development of minimally-invasive solutions for symptomatic uterine fibroids, today announced that it has received 510(k) clearance from the FDA to market its Sonata® Sonography-Guided Transcervical Fibroid Ablation (Sonata) System.
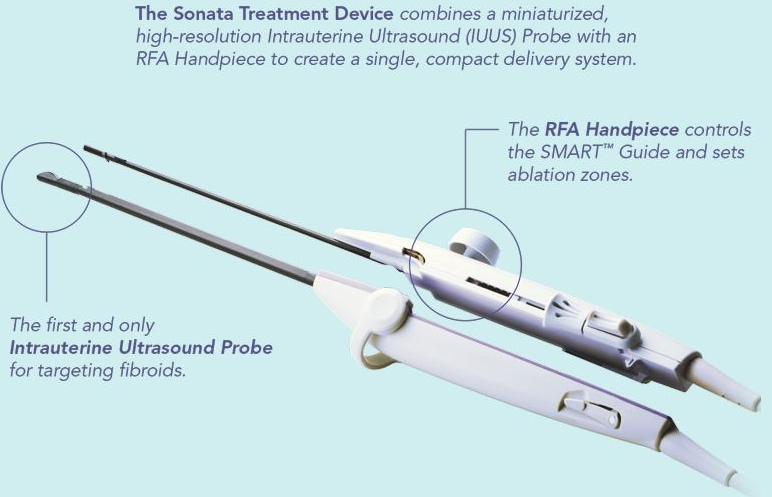
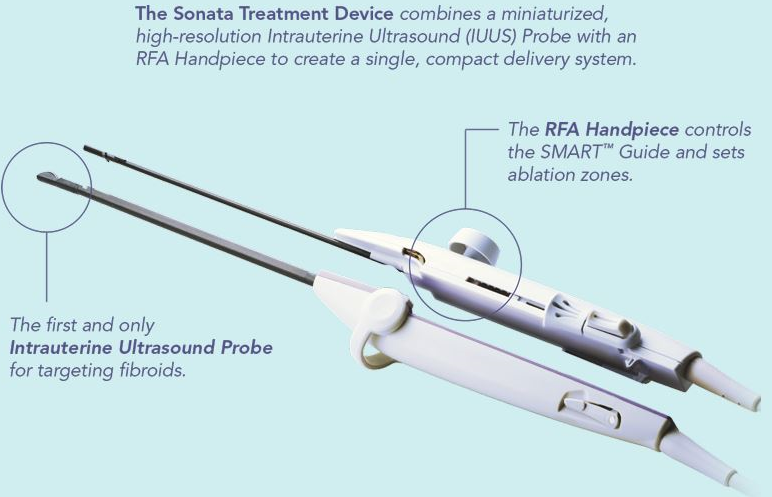
The Sonata System combines a breakthrough integrated technology which is the first and only intrauterine ultrasound system with a proprietary radiofrequency ablation device, providing a transcervical, incision-free, uterus-preserving treatment for uterine fibroids.
With an estimated volume of more than 1 million annual global fibroid procedures, Gynesonics projects
a $3 billion-4 billion global market opportunity for its Sonata System for the treatment of uterine fibroids, including a market opportunity of more than $1 billion in the U.S. alone.
“We are excited to announce FDA clearance of our Sonata System. This is an essential milestone for
Gynesonics in our mission to bring this transformational technology for the treatment of uterine fibroids
to women,” said President and CEO Christopher Owens. “The patient outcomes achieved with Sonata
demonstrate its potential to become a new standard of care in fibroid treatment providing important
patient benefits as compared to invasive surgery. Clinical trial outcomes show that Sonata provides a
significant and durable improvement in symptoms with more than half the women returning to normal
activities within 24 hours.”
In a published survey of 968 women affected by fibroids (American Journal of Obstetrics and
Gynecology, 2013) the key findings include:
● 79% of women want to avoid invasive surgery;
● Affected women wait an average of 3.6 years before being treated;
● Most women, regardless of desire for future fertility, want to preserve their uterus.
“The published survey results show that women are looking for new options to address this widespread
condition, they want to preserve their uterus and they wish to avoid invasive surgery, including hysterectomy,” Owens added. “Sonata is the only system that can treat the vast majority of all fibroids
with a transcervical, incision-free, uterus-preserving approach. Current transcervical options are limited
to treatment of a small portion of the total fibroid market by comparison.”
According to medical literature, about 70-80 percent of women in the U.S. will develop uterine fibroids
by age 50, with a significant proportion of women having symptoms, many of which can be severe and
debilitating. In addition, according to the New England Journal of Medicine, approximately 200,000
hysterectomies are performed in the United States each year because of symptomatic fibroids.
Owens added that the company exceeded the FDA-specified 12-month primary endpoint performance
requirements for the Sonata System in the SONATA pivotal IDE clinical trial used to support the FDA
regulatory 510(k) filing. The co-primary endpoints included a significant reduction in bleeding and a
high rate of freedom from surgical reintervention due to heavy menstrual bleeding (HMB). The safety
outcomes were also favorable, including zero device related adverse events reported in the 147-patient
trial.
About Sonata System
The Sonata System, the next generation of Gynesonics’ technology platform (the previous generation
referred to as VizAblate), uses radiofrequency energy to ablate fibroids under intrauterine sonography
guidance. The Sonata System, including the SMART Guide™, enables the operator to target fibroids
and optimize ablations within them. Sonata system’s design provides a straightforward, transcervical
access for a uterus preserving, incision-free fibroid treatment. This intrauterine approach is designed to
avoid the peritoneal cavity.
Read More at the Source: Press Releases – Gynesonics (US)
Press Release by Gynesonics
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.