Medical device and MedTech insights, news, tips and more
Varian Medical Systems Receives FDA 510(k) Clearance for Halcyon Treatment System
July 20, 2017
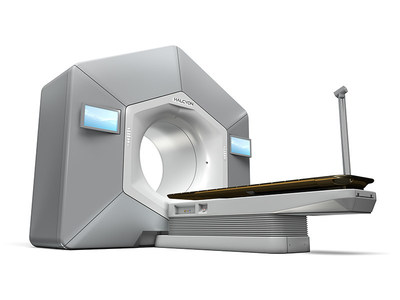
PALO ALTO, Calif., June 29, 2017 /PRNewswire/ — Varian Medical Systems (NYSE: VAR) has received FDA 510(k) clearance for its Halcyon™ system, its new device for cancer treatment. Halcyon simplifies and enhances virtually every aspect of image-guided volumetric intensity modulated radiotherapy (IMRT).
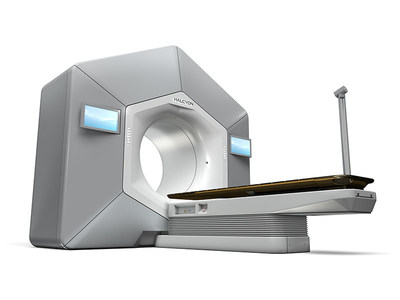
“We are proud that Halcyon has now received both 510(k) clearance and CE mark,” said Kolleen Kennedy, president of Varian’s Oncology Systems business. “With its human-centered and user-friendly design, Halcyon is engineered to revolutionize clinical workflow. These two clearances are very important milestones in the availability of this new system and advancing cost-effective cancer care worldwide.”
Halcyon is an advanced system that is more comfortable for the patient while delivering ease of use for healthcare providers, accelerated installation timeframes, expedited commissioning, simplified training, and automated treatment. With its streamlined workflow, the system only requires nine steps from the start to the end of treatment compared to up to more than 30 steps with older technologies. Halcyon is well suited to handle the majority of cancer patients, offering advanced treatments for prostate, breast, head & neck, and many other forms of cancer.
Halcyon features large touchscreens on both sides of the machine to assist in easy patient set-up. For increased patient comfort, Halcyon is up to 2x quieter than other systems, has a low couch height for easy patient access, and soft indirect ambient lighting in the gantry opening. To create a closer connection between patient and therapist during Halcyon treatment, the system includes an integrated couch-mounted camera for the therapist to watch over the patient during treatment, and an integrated noise-cancelling sound system that makes it easy for patients to converse with the therapists.
With 510(k) clearance, Varian can begin selling the Halcyon system in the United States. The system requires Eclipse™ 15.1.1 treatment planning software, which is currently 510(k) pending. For more information on Halcyon visit www.varian.com/halcyon.
About Varian Medical Systems
Varian Medical Systems focuses energy on saving lives and is the world’s leading manufacturer of medical devices and software for treating and managing cancer. Headquartered in Palo Alto, California, Varian employs approximately 6,400 people around the world. For more information, visit http://www.varian.com and follow @VarianMedSys on Twitter.
Source: Varian Medical Systems Receives FDA 510(k) Clearance for Halcyon Treatment System – Jun 29, 2017
