Medical device and MedTech insights, news, tips and more
Versa Capital Sets Their Eyes on SynCardia Systems, Creator of the Only FDA-approved Artificial Heart
July 8, 2016
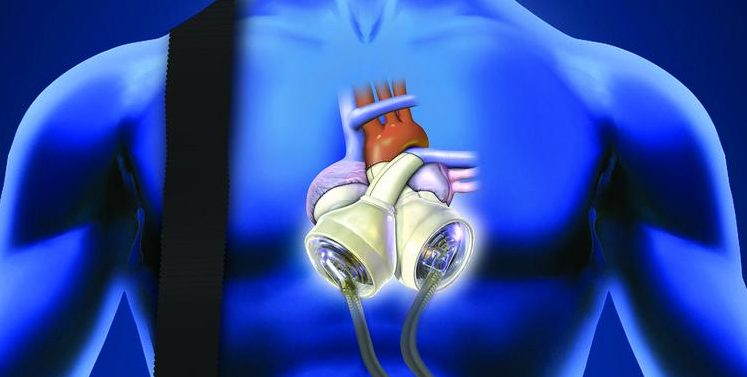
Artificial heart maker SynCardia Systems has filed for Chapter 11 bankruptcy reorganization with plans to sell all of its assets to a Philadelphia-based private-equity firm, according to reports in the Arizona Daily Star .
Tucson-based SynCardia, maker of the world’s only U.S. Food and Drug Administration-approved artificial heart, will continue operations as an affiliate of Versa Capital Management LLC seeks court approval to buy the assets out of bankruptcy and recapitalize the company.
SynCardia announced July 2 that it had entered into the asset-purchase agreement with an affiliate of Versa to acquire substantially all of the company’s assets and operations.
The company’s day-to-day operations will continue without interruption during the acquisition, according to information about the acquisition on the Arizona Bioindustry Association website.
“Our first priority has been and will always be the relationship with our hospitals and the care of their many patients, none of which will be adversely effected by this sale process,” said Michael Garippa, CEO of SynCardia, in a statement. “We have developed a transaction structure to free the organization from substantial liabilities and maximize the value of our business.”
Garippa said the sale provides a “strong foundation” for growth and development of SynCardia’s next-generation heart driver system, the Freedom 2.
“We are acting to ensure that SynCardia will be in the best position to provide lifesaving treatments for end-stage heart failure and to adapt our business to meet the changing needs of the health care industry,” Garippa said in the statement.
Read the Full Article – Source: Versa Capital to acquire SynCardia Systems, maker of the world’s only FDA-approved artificial heart – Phoenix Business Journal

