Medical device and MedTech insights, news, tips and more
VivaLNK Announces World’s First FDA Cleared Wearable ECG Sensor Platform
January 23, 2020
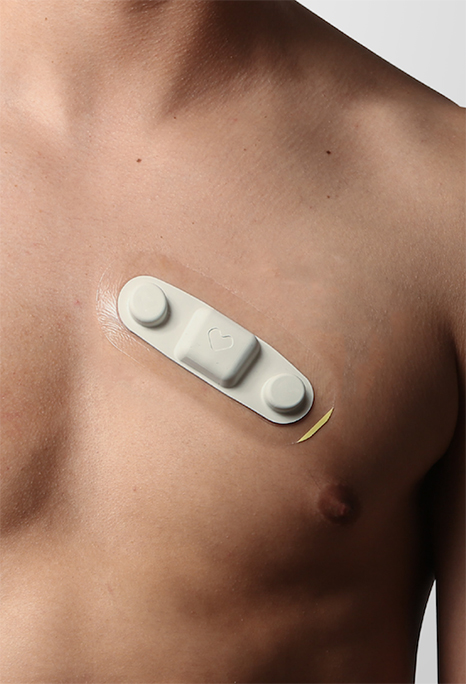
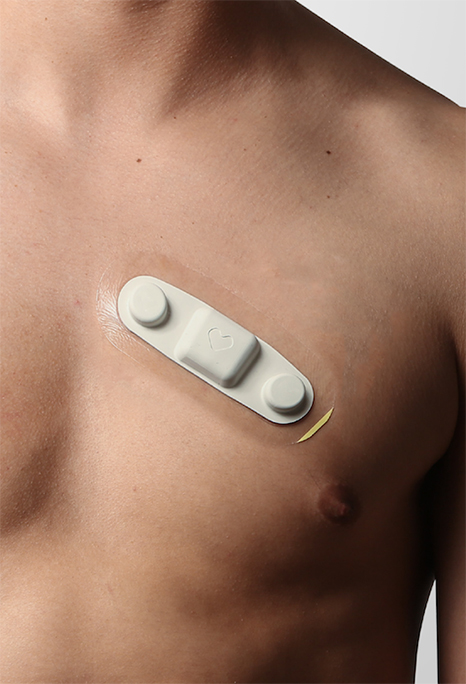
VivaLNK, a leading provider of connected healthcare solutions, today announces it received FDA clearance for its Continuous ECG Platform. Consisting of reusable wearable ECG sensors and associated software development kit (SDK), the sensor platform gives developers and providers direct control over data, and represents the first of its kind to receive FDA clearance.
One of the key issues surrounding medical IoT solutions is data control – who owns it and where it is stored. Most wearable medical devices that offer remote access force application developers and solution providers to use a 3rd party cloud for access.
“Caretaker is pleased to select VivaLNK as the FDA 510k cleared ECG sensor provider for our continuous wireless vital signs platform and thrilled to have a best-in-class form factor that is incredibly comfortable for the patient, easy to integrate into our system, reliable for our healthcare provider customers, and allows direct control of the data flow into our secure CaretakerCloud remote patient monitoring portal,” said Jeff Pompeo, President and CEO of Caretaker Medical, Charlottesville, Virginia.
With the VivaLNK platform, application developers can integrate with VivaLNK medical wearable sensors, and send that data directly to the application. In addition, the VivaLNK sensor does not collect personally identifiable information.
“VivaLNK’s Continuous ECG Platform has been seamlessly integrated with the AMPS Continuous ECG Recording Suite (CER-S) allowing for the direct analysis of the recorded data, and can therefore be immediately adopted by the pharmaceutical industry in the context of clinical trials,” said Fabio Badilini, President and Chief Scientist at AMPS.
The SDK in the platform not only allows developers to integrate directly with the wearable ECG sensor, but also integrates with the previously FDA cleared and CE Marked VivaLNK temperature sensor. Thus, enabling multiple vitals to be remotely monitored from a single platform.
See Full Press Release: VivaLNK Announces World’s First FDA Cleared Wearable ECG Sensor Platform
Written by: VivaLNK

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.