Medical device and MedTech insights, news, tips and more
Wireless Sensor-Enabled Spine Implant Company, Intellirod Spine, Raises Over $1M from Investors
August 3, 2016
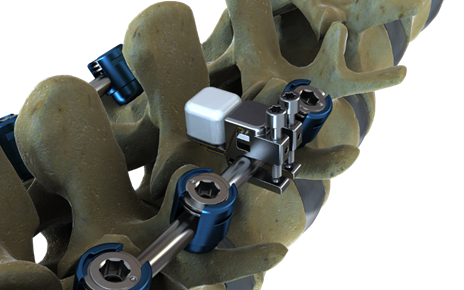
Akron, Ohio-based Intellirod Spine, a sensor-enabled spine implant company, has raised more than $1 million in a round led by new and existing investors, including Queen City Angel First Fund V and JumpStart. Previous investors include the Kentucky Seed Capital Fund and the City of Akron, Ohio.
Intellirod Spine is developing wireless sensors that will measure the strain being put on implanted spinal fusion rods. Data from these sensors, which use RFID technology, will help surgeons monitor the post-operative progress of patients.
The company is currently developing two products: Loadpro, an intra-operative sensor for use in monitoring rod strain during kyphotic correction surgery, and Accuvista, an implantable sensor that measures post-operative strain in rods in degenerative disc disease fusion patients. With both devices, the doctor uses a special reader device to collect data from the sensors.
“With Loadpro, for the first time, spine surgeons will have an accurate tool to measure and determine the amount of force being applied to the rod implant as spinal deformity is being corrected,” Dr. Rolando Pulo, the founder of the company, said in a statement. “Knowing the amount of applied strain will allow spine surgeons to adjust their surgical techniques to achieve the best possible correction of deformity. Furthermore, spine surgeons will be able to avoid the risk of inadvertently applying excessive strain causing fixation failure and/or loss of correction.”
Intellirod Spine is currently pursuing de novo FDA clearance and a CE Mark for Loadpro, a process that involves ongoing clinical studies in Louisville, Kentucky and Columbus, Ohio, with a third set to begin in Cleveland. Accuvista is also awaiting FDA clearance, but has completed pre-clinical testing.
“The Accuvista sensor can be a game-changer for patients undergoing posterior spinal fusion for a wide range of spine conditions including those from deformity, degenerative disease, and trauma,” Puno said. “Accuvista allows a spine surgeon to be able to accurately determine when fusion has occurred without the need of CAT scans. CAT scans are costly, expose the patient to significant radiation, and are only done periodically as a ‘snapshot in time’. Accuvista is a real time monitor. Accuvista provides the doctor with an accurate basis for advising post-spinal fusion patients on when it will be safe for them to return to normal physical activity and/or work.”
Source: Intellirod Spine raises $1M to add wireless sensors to spine implants | MobiHealthNews
