Medical device and MedTech insights, news, tips and more
Zimmer Biomet Receives FDA Clearance for ROSA® Knee System for Robotically-Assisted Surgeries
January 25, 2019
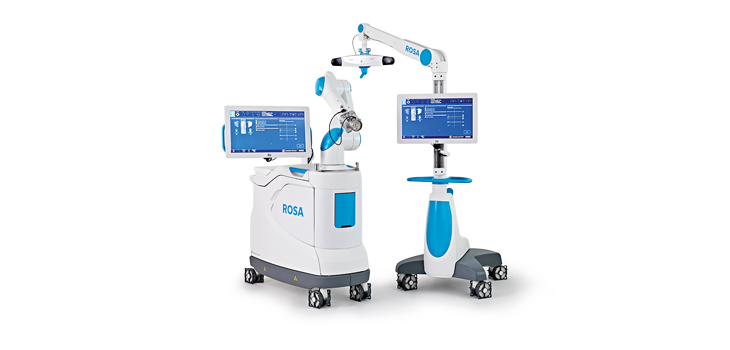
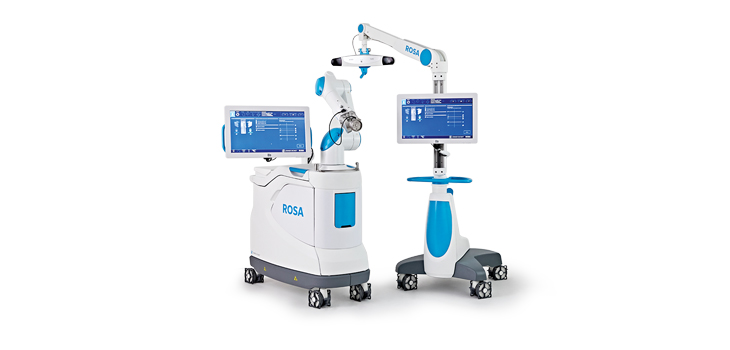
Zimmer Biomet Holdings, Inc., a global leader in musculoskeletal healthcare, today announced U.S. Food and Drug Administration 510(k) clearance of the ROSA® Knee System for robotically-assisted total knee replacement surgeries. ROSA Knee features 3D pre-operative planning tools and real-time, intraoperative data on soft-tissue and bone anatomy designed to improve bone cut accuracy and range of motion gap analysis to potentially improve flexion and restoration of natural joint movement.
“Complementing the skill and expertise of the surgeon with ROSA Knee’s robotically-assisted technologies can improve accuracy, precision and consistency, which can improve patient satisfaction, clinical outcomes and efficiency,” said Christopher J. Cannova, M.D., Washington Joint Institute at OrthoBethesda. “ROSA Knee functions as a surgical assistant that gives me the tools and real-time data to perform bone cuts with greater precision and improve patient-specific soft-tissue balancing and implant alignment, without losing my feel for a natural fit and flexion.”
ROSA Knee leverages Zimmer Biomet’s ROSA Robotics platform, which includes ROSA Brain for neurosurgical procedures.
“We are excited for the launch of ROSA Knee, which brings together Zimmer Biomet’s robotics technology with our industry-leading Knee implants to help surgeons personalize surgical procedures for their patients,” said Ivan Tornos, Group President, Orthopedics. “Zimmer Biomet is committed to leading the industry in bringing differentiated and holistic solutions to market that address the needs of our customers and improve patient outcomes.”
See Full Press Release at the Source: Zimmer Biomet Receives FDA Clearance for ROSA® Knee System for Robotically-Assisted Surgeries
Press Release by Zimmer Biomet
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.