Medical device and MedTech insights, news, tips and more
In-Home Fertility Tracking System Wins CE Mark
November 1, 2018
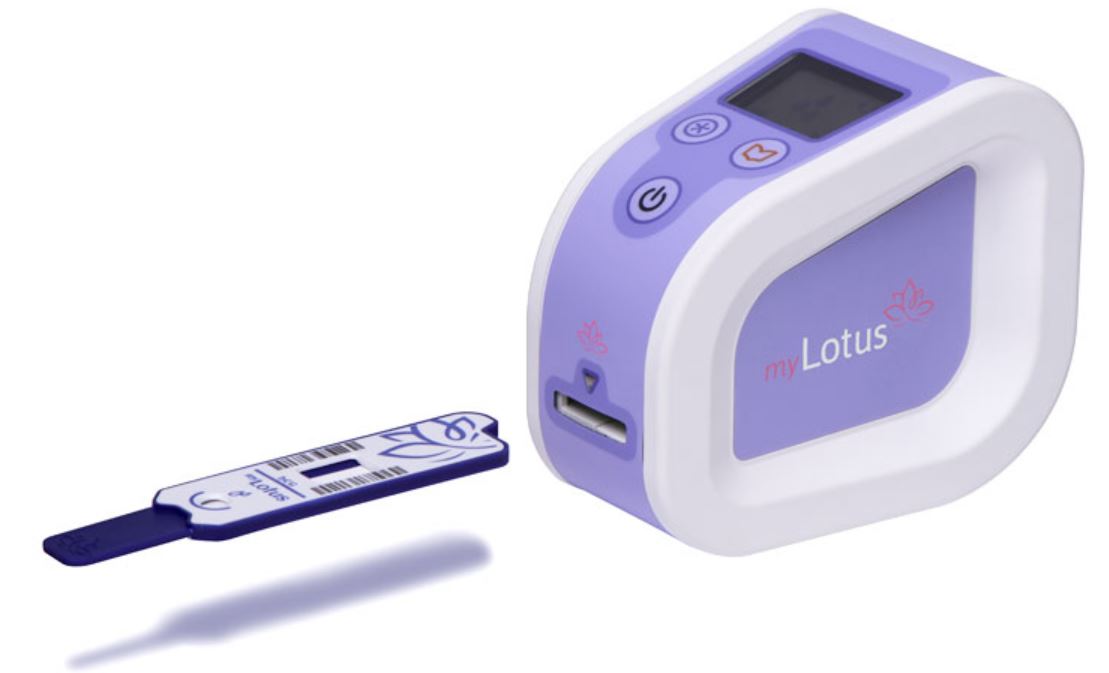
Concepta plc, the innovative UK healthcare company and developer of the proprietary self-test platform (“myLotus”) and suite of emerging test products targeting the mobile health market is pleased to announce it has received CE-Mark certification for its myLotus branded products for women’s fertility.
Given the stringent regulatory environment for diagnostics, CE certification is a major milestone enabling the Company to sell its breakthrough fertility test device ‘myLotus’ in the UK market and subsequently across CE-Mark territories.
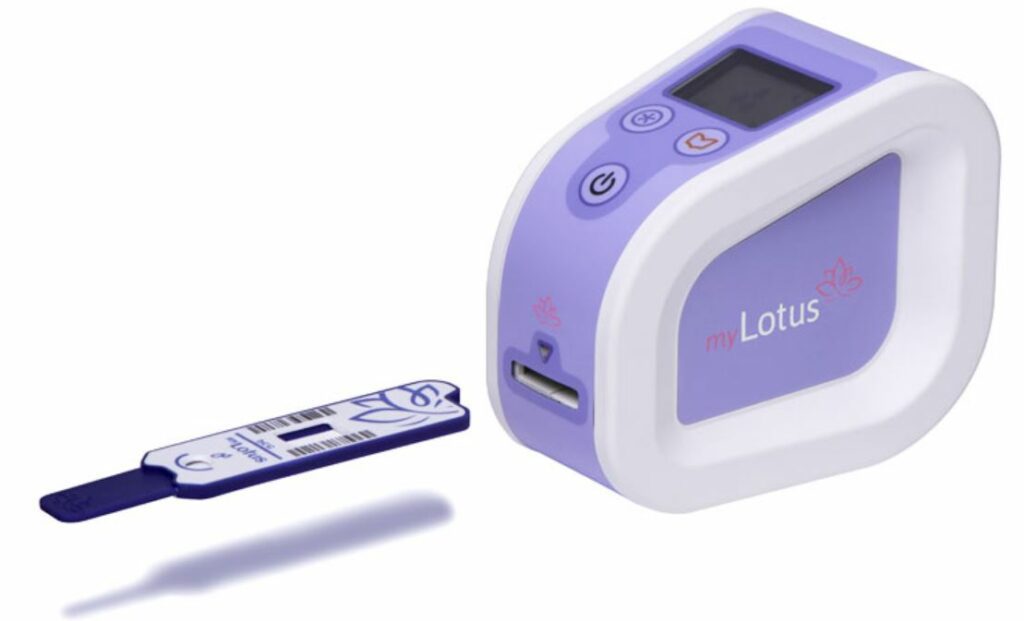
myLotus allows women the ability to self-test to identify their optimal fertile days thereby improving their chances of naturally getting pregnant. myLotus enables women to monitor their fertile phase as part of their menstrual cycle and is the only home test to ‘quantitatively’ measure personalised hormone levels and the ‘rate-of-change’ of these levels preceding ovulation to help aid a natural conception. Users measure, record and track their hormone levels and follow their fertility journey in an accompanying free downloadable app.
The Company expects to launch its new fertility products under the brand name ‘myLotus’ with initial demand serviced via the Company’s new eCommerce platform and website mylotus.com
The product will be available to women who are planning to start a family or for those who have previously struggled to naturally conceive, enabling couples an alternative prior to considering more costly IVF treatment.
Matthew Walls, Chairman said: “following several years of diligent work by our technology team, study doctors and trial users we are delighted to receive CE-Mark certification for our first myLotus products. Novel self-test diagnostics for home-use is a highly regulated field of healthcare and this is a fabulous and eagerly awaited endorsement for the Company. Importantly the certification signals our drive to help improve the chances of women naturally getting pregnant and our preparations for the launch of myLotus in the UK and other CE-Mark territories. We will advise on the timing of the UK launch very shortly.”
See Full Press Release at the Source: IR Solutions, Euroinvestor
Press Release by: Concepta plc
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
