Medical device and MedTech insights, news, tips and more
Aquablation Therapy is the first surgical robotics system to receive a MedTech Innovation Briefing (MIB) from the National Institute for Health Care Excellence
March 9, 2023
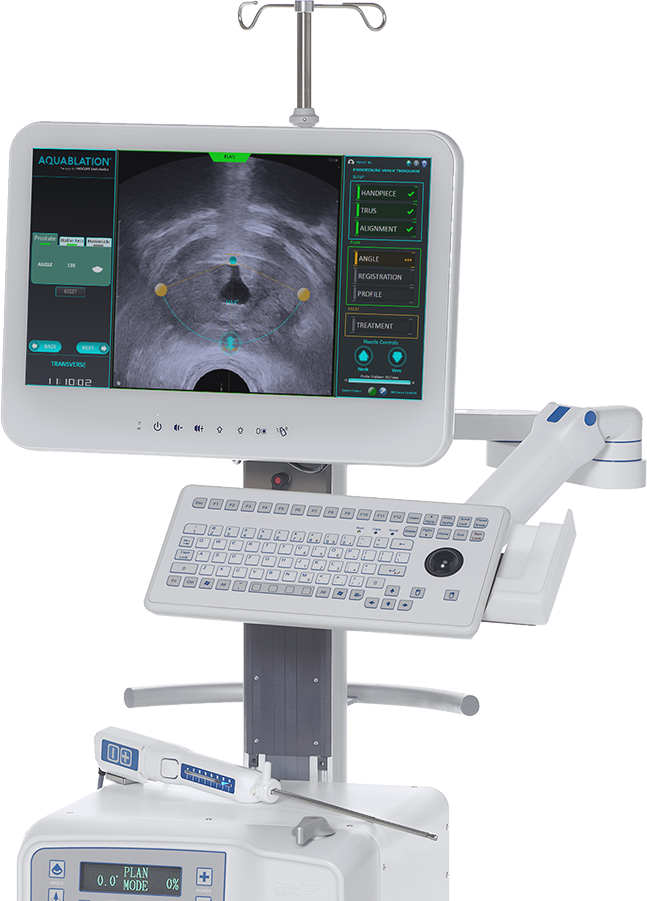
PROCEPT® BioRobotics Corporation today announced Aquablation Therapy received a MedTech Innovation Briefing (MIB) from the National Institute for Health Care Excellence (NICE), for benign prostate hyperplasia (BPH) in the United Kingdom. NICE has recognized Aquablation Therapy is as effective as transurethral resection of the prostate (TURP) for the removal of prostate tissue for men with BPH. A panel of clinical experts said the technology is innovative compared to the standard of care and offers additional benefits, such as increased ability to preserve sexual function. The clinical experts associated with the review stated that the technology has the potential to replace TURP and will challenge holmium laser enucleation for larger prostates.
Aquablation therapy is the first and only robotic surgical system to receive a MIB. MIBs are designed to support decision-making by health care professionals about the adoption and impact of new technology through detailed technical, clinical and economic assessments. New technology is selected on the basis of high demand on the topic, the potential for useful clinical outcomes, and whether evidence is available.
Mr. Neil Barber, one of the Briefings’ Clinical Experts and a PROCEPT BioRobotics consulting physician, stated, “We have been treating men successfully with Aquablation therapy for over seven years and believe it will soon become the standard of care for BPH. Patients ultimately want their work, leisure, and sex lives back without risking long-term catheterization or loss of sexual function. AquaBeam’s real time image guidance allows surgeons to see a patient’s anatomy through a multidimensional view, allowing for a fully customized treatment plan. The robot executes the treatment plan and guides the waterjet with speed and accuracy leading to a more consistent, predictable, and safer patient outcome. Moreover, it is much easier to train new urologists to become proficient using Aquablation therapy, which bodes well for the future.”
“The NICE MedTech Innovation Briefing further validates the value we believe Aquablation Therapy brings to patients, doctors, hospitals and healthcare systems around the world”, said Sham Shiblaq, Executive Vice President, Chief Commercial Officer of PROCEPT® BioRobotics. “The robust body of clinical evidence shows Aquablation therapy offers improved safety and durability when compared to transurethral resection of the prostate, which is the current gold standard surgical treatment option.”
About PROCEPT BioRobotics Corporation
PROCEPT BioRobotics is a surgical robotics company focused on advancing patient care by developing transformative solutions in urology. PROCEPT BioRobotics develops, manufactures and sells the AquaBeam Robotic System, an advanced, image-guided, surgical robotic system for use in minimally invasive urologic surgery with an initial focus on treating benign prostatic hyperplasia, or BPH. BPH is the most common prostate disease and impacts approximately 40 million men in the United States. PROCEPT BioRobotics designed Aquablation therapy to deliver effective, safe and durable outcomes for males suffering from lower urinary tract symptoms, or LUTS, due to BPH that are independent of prostate size and shape or surgeon experience. The Company has developed a significant and growing body of clinical evidence, which includes nine clinical studies and over 150 peer-reviewed publications, supporting the benefits and clinical advantages of Aquablation therapy.
See Full Press Release at the Source: Aquablation Therapy is the first surgical robotics system to receive a MedTech Innovation Briefing (MIB) from the National Institute for Health Care Excellence
Press Release by: PROCEPT® BioRobotics
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

