Medical device and MedTech insights, news, tips and more
Avails Medical’s eQUANT™ System Submitted to FDA for 510(k) Clearance
June 12, 2023
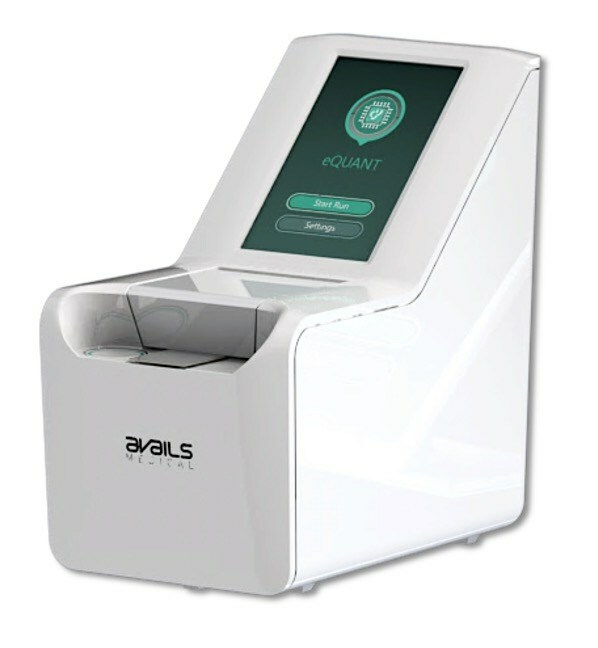
Avails Medical, a pioneer in rapid, automated and fully electrical antibiotic susceptibility testing (AST) solutions announced today the submission of its eQUANTTM system for FDA 510(k) clearance.
The eQUANT system provides a standardized inoculum (0.5 McFarland equivalent) directly from a positive blood culture, which is designed to be used with traditional automated AST systems and Disk Diffusion, accelerating routine AST turnaround times by up to one day.
“The global emergence of superbugs and rise in antimicrobial resistance is a leading public health concern. Providing clinical laboratories with rapid diagnostics is key to address this important challenge. eQUANT could have the potential to enable physicians to de-escalate from costly empiric, broad-spectrum antibiotics to an effective and lower cost therapy, a real game changer for patients,” said Dr. Niaz Banaei, Professor of Pathology and Medicine and Medical Director, Stanford Health Care Clinical Microbiology Laboratory and investigator in the clinical study.
The eQUANT system’s unique features include a very small footprint, easy-to-use user interface and affordable economics, making it well suited for widespread adoption. In a previous pilot study completed at two world-renowned clinical laboratories, the eQUANT system achieved >98% correlation when compared to the subculture gold standard.
“We are delighted to announce the important company milestone of FDA submission of the highly anticipated eQUANT system”‘ said Dr. Knopfmacher, CEO of Avails Medical. “This is an important first step towards commercializing eQUANT in the U.S. and other countries. eQUANT can provide any clinical laboratory with a simple and powerful tool that is designed to fit seamlessly into the routine clinical laboratory’s workflow, accelerating traditional AST turnaround times by up to one day.” Avails Medical will showcase the eQUANT System as well as the eASTTM System at the upcoming ASM conference in Houston in June (booth 1814).
In addition to FDA submission, Avails Medical also announced today an additional $5.4M million in non-dilutive funding from CARB-X for its rapid eAST system, after successfully achieving additional gating milestones. The Combating Antibiotic Resistant Bacteria Biopharmaceutical Accelerator (CARB-X), is a global non-profit partnership based at Boston University dedicated to funding the development of new antibiotics, vaccines, diagnostics, and other products to address the rising global threat of antibiotic-resistant bacteria.
Contacts:
press@availsmedical.com
About Avails Medical, Inc.
Avails Medical was founded to address one of our greatest global health threats: antibiotic resistance. The Avails all-electrical technology is designed to significantly reduce the amount of time required to obtain the reliable antibiotic susceptibility data required to enable accurate therapy decisions. www.availsmedical.com
See Full Press Release at the Source: Avails Medical’s eQUANT™ system submitted to FDA for 510(k) clearance
Press Release by: Avails Medical
Legacy MedSearch has more than 35 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 17 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

