Medical device and MedTech insights, news, tips and more
Axonics® Announces U.S. Food & Drug Administration Approval for its Sacral Neuromodulation System
September 9, 2019
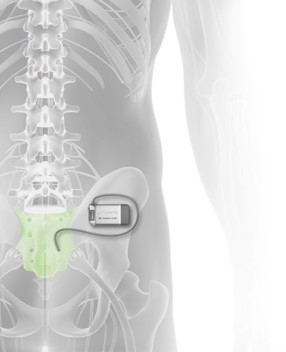
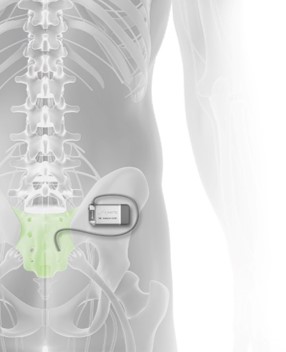
Axonics Modulation Technologies, Inc. (NASDAQ: AXNX), a medical technology company that has developed and is commercializing a novel implantable rechargeable sacral neuromodulation (“SNM”) device for the treatment of urinary and bowel dysfunction, today announced the approval of the Axonics r-SNM® System by the United States Food & Drug Administration (“FDA”). The Axonics System is the first rechargeable SNM system approved for sale in the United States, Europe, Canada and Australia.
The FDA approval grants Axonics the right to market its product in the United States for the clinical indication of fecal incontinence. The approval includes the claim of a 15-year functional life and the ability of patients to undergo full-body MRI scans without the necessity of having the device explanted.
Axonics has an additional pre-market approval (“PMA”) filing currently under review with the FDA for the clinical indications of overactive bladder (urinary urgency incontinence and urinary urgency frequency) as well as urinary retention for which the Company anticipates a determination in the near term.
Raymond W. Cohen, CEO of Axonics, commented, “If we consider the millions of women who are suffering in silence with bladder and bowel dysfunction, we believe the market opportunity for Axonics goes well beyond the existing approximately $650 million of revenue that is currently being generated by the incumbent’s non-rechargeable SNM device. We believe the number of patients seeking SNM treatment will expand dramatically over the next few years given our fuss-free, long-lived, full body MRI-compatible device. In the months prior to FDA approval, we invested significant time and resources in building inventory to support our fully trained, U.S. commercial team which now includes 145 territory managers, clinical support specialists and sales managers strategically located around the country. We plan to begin shipping product to U.S. physicians and hospitals during the fourth quarter of 2019, following the fulfillment of customary, pre-launch regulatory requirements.”
About Sacral Neuromodulation and the Axonics r-SNM System
Sacral neuromodulation is used to treat bladder and bowel dysfunction. These conditions are caused by a miscommunication between the bladder and the brain and significantly impacts quality of life. Overactive bladder affects an estimated 87 million adults in the U.S. and Europe. Another estimated 40 million adults are reported to suffer from fecal incontinence/accidental bowel leakage. SNM therapy has been employed to reduce symptoms and restore pelvic floor function for the past two decades. Reimbursement coverage is well established in the U.S. and Europe. The Axonics r-SNM System is the first rechargeable SNM system approved for sale in the world, and the first to gain full-body MRI conditional labeling. The Axonics r-SNM System offers a long-lived miniaturized neurostimulator that is approximately the size of a USB stick and is qualified to last 15 years in the body as compared to the incumbent’s device which is non-rechargeable and requires patients to undergo a surgical intervention to replace the device every 3 to 5 years. In addition to full body MRI conditional labeling, the Axonics r-SNM System features many differentiating attributes. These include a patented tined lead, user-friendly accessories, a wireless charging system optimized for infrequent charging, a small easy-to-use patient remote control and an intuitive clinician programmer that facilitates lead placement and stimulation programming.
PMA approval process with the U.S. FDA
Determination by the FDA of the safety and effectiveness of the Axonics r-SNM System was supported by the results of a detailed review of technical data, published SNM clinical literature and the ARTISAN-SNM 129-patient pivotal clinical study. ARTISAN-SNM was conducted at 14 centers in the U.S. and five centers in Western Europe and met all primary and secondary endpoints. No serious device-related adverse events were reported. During the review period the FDA conducted two detailed PMA audits of the Axonics quality system and manufacturing. The audits were completed without any negative observations.
See Full Press Release: Axonics® Announces U.S. Food & Drug Administration Approval for its Sacral Neuromodulation System | Axonics Modulation Technologies, Inc.
Press Release Written By: Axonics Modulation Technologies

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.