Medical device and MedTech insights, news, tips and more
Baxter Launches Peri-Strips Dry With Secure Grip Technology for Reliable Staple Line Reinforcement in Surgical Procedures
March 2, 2020
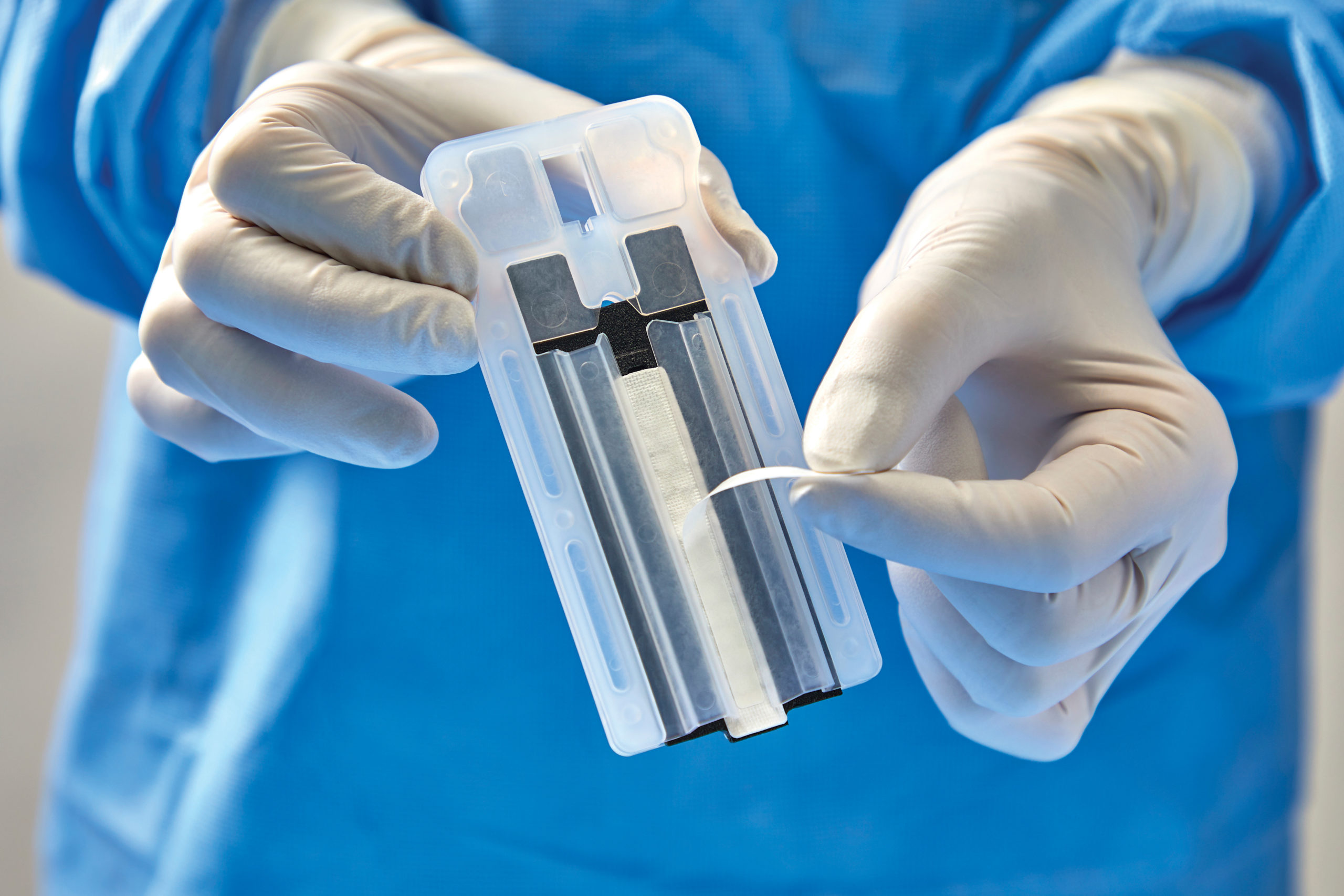
Baxter International Inc., a global leader in advancing surgical innovation, announced 510(k) clearance from the U.S. Food and Drug Administration for a new generation of its Peri-Strips Dry with Veritas Collagen Matrix (PSDV) product, known as PSDV with Secure Grip. Now available with “peel and secure” technology, PSDV is a staple line reinforcement material that bariatric surgeons have used to mitigate staple line complications for more than 15 years. This new generation of PSDV is two times faster to prepare compared to the previous version and another staple line reinforcement product.1
“We continue to advance innovation by providing surgeons tools that are faster and easier to use in the operating room,” said Wil Boren, president of Baxter’s Advanced Surgery business. “As surgical procedures are becoming more complex, it is important that surgeons have the tools they need to optimize patient care.”
Despite the sophistication of modern stapling technology used in bariatric procedures, staple line complications that result in high morbidity and mortality persist. The most prevalent complications are staple line bleeding and leaks. While the incidence of these complications is low, the impact can be clinically debilitating for the patient and costly to address.2
PSDV is proven to effectively reduce intraoperative and postoperative staple line bleeds and leaks in routine bariatric procedures such as a sleeve gastrectomy or gastric bypass. A 2015 meta-analysis extracted data from 295 studies predominantly comprised of morbidly obese patients undergoing laparoscopic gastric bypass and laparoscopic sleeve gastrectomy to include nearly 42,000 patients concerning bleeds and over 56,000 patients concerning leaks. PSDV showed statistically significant greater relative efficacy in reducing bleeding and leaks when compared to no staple line reinforcement, oversewing, and another staple line reinforcement product.2
PSDV with Secure Grip is applied to the surgical stapler via a pressure-sensitive adhesive strip. The new generation of PSDV provides strong adherence to the stapler, allowing surgeons to easily manipulate tissue. In a recent Staple Line Reinforcement Comparison study,1 30 bariatric nurses and surgical technicians evaluated stapler preparation time, ease of use and learnability of the PSDV with Secure Grip versus other options. Participants unanimously chose PSDV with Secure Grip as the most preferred staple line reinforcement material in the operating room.
See Full Press Release: Baxter Launches Peri-Strips Dry With Secure Grip Technology for Reliable Staple Line Reinforcement in Surgical Procedures | Business Wire
Written by: Baxter

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

