Medical device and MedTech insights, news, tips and more
Boston Scientific Recalls Lotus Valve System Units for Delivery System Malfunction
August 22, 2016
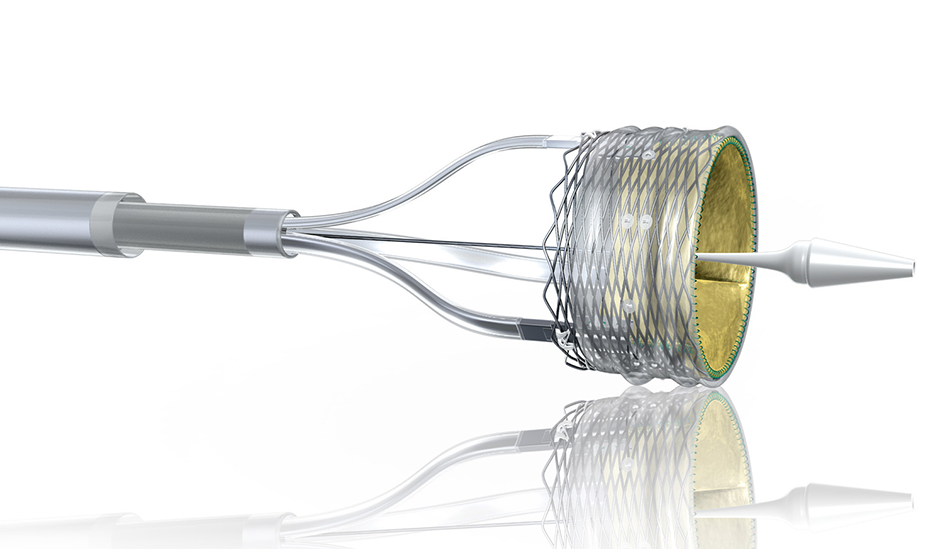
MARLBOROUGH, MA — Boston Scientific has issued a voluntary global recall for several units of the Lotus Valve System because of breakage issues with the release mandrel, a component of the delivery system.

Release mandrel breaks most commonly resulted in the need to resheath and remove the device, which added to procedural times, but also resulted in three deaths due to catastrophic vessel trauma, according to a statement by the company. The deaths occurred in Europe between February and June 2016, company officials told heartwire from Medscape.
The release mandrel is connected to the release pin, which facilitates release of the transcatheter valve from the delivery system.
A change was made to the component in March 2016 and no new reports of release mandrel breakage have been received since then, according to the company.
It also notes that there are no additional safety issues for patients who previously received a Lotus implant, since the defect involves the delivery system and is not related to the performance of the implanted valve.
The Lotus valve was approved in Europe in October 2013 and is being evaluated for US approval in the ongoing REPRISE III trial.
Boston Scientific is asking physicians to remove and return all affected units. Approximately 250 affected devices are out in the field worldwide. The UPNs cited in the urgent field safety notice are H749LTV230, H749LTV250, and H749LTV270. A full list of the affected lots is available here.
In November 2014, Boston Scientific issued another recall for 276 units of the Lotus Valve System (H749LTV230) distributed in Europe because the valve became unlocked during release from the delivery system.
Read Full Article – Source: Boston Scientific Recalls Some Lotus Valve Systems for Delivery System Malfunction
Author – Patrice Wendling
Photo Credit – Boston Scientific
