Medical device and MedTech insights, news, tips and more
CardioFocus Announces US FDA Approval of HeartLight X3 System for the Treatment of Atrial Fibrillation
May 12, 2020
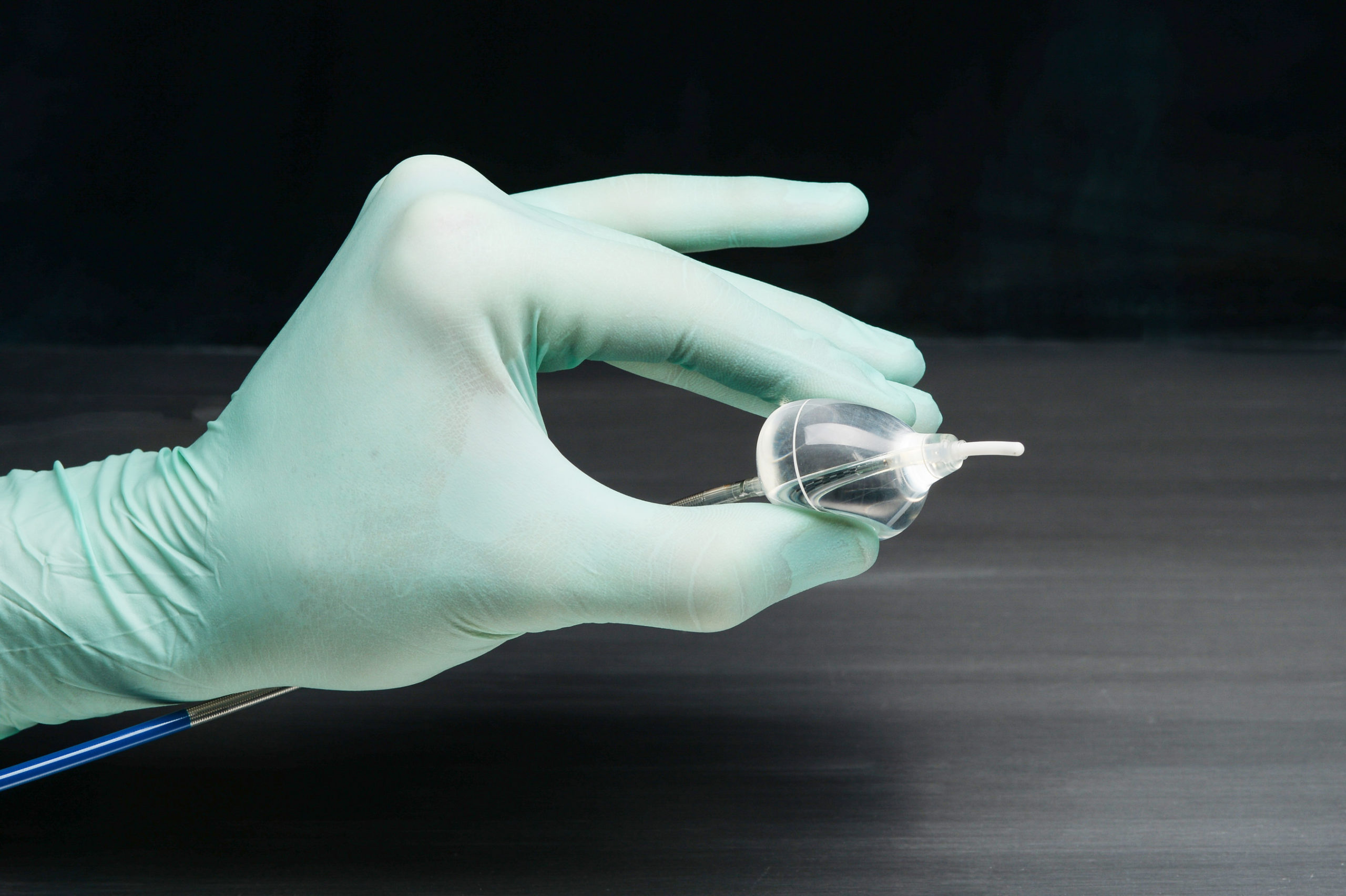
CardioFocus, Inc. today announced that the U.S. Food and Drug Administration (FDA) has approved the next-generation HeartLight® X3 Endoscopic Ablation System for the treatment of drug refractory recurrent symptomatic paroxysmal atrial fibrillation (PAF).
Approval of the HeartLight X3 System came as a result of a comprehensive submission, including outcomes from the study of 60 HeartLight X3 patients. In this pivotal confirmatory study, the X3 System achieved very rapid pulmonary vein isolation (PVI), in as few as three minutes for a single pulmonary vein.
“The HeartLight X3 System introduces a new level of speed, control and predictability for physicians – making it an ideal tool for AFib ablation,” said Vivek Y. Reddy, M.D., Director of Cardiac Electrophysiology and Helmsley Trust Professor of Medicine at The Icahn School of Medicine at Mount Sinai. “This is a transformational technology when it comes to AFib ablation. Introducing direct visualization of the pulmonary veins and combining it with the ability to create continuous lesions is compelling.”
“FDA approval of the HeartLight X3 System represents a substantial milestone for CardioFocus and is a promising new treatment option for the millions of Americans suffering from paroxysmal AFib,” said Burke T. Barrett, Chief Executive Officer at CardioFocus. “By pairing the most compliant and dynamic balloon technology with the ability to deploy titratable laser energy at unprecedented speed while using direct visualization, we are able to offer a completely unique ablation technology to cardiac electrophysiologists. With this approval in hand, we will initiate a focused rollout of HeartLight X3 across the US.”
See Full Press Release: CardioFocus Announces US FDA Approval of HeartLight® X3 System for the Treatment of Atrial Fibrillation
Written by: CardioFocus

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

