Medical device and MedTech insights, news, tips and more
Edwards Lifesciences gets approval for SAPIEN 3 TAVI valve in Europe
November 14, 2019
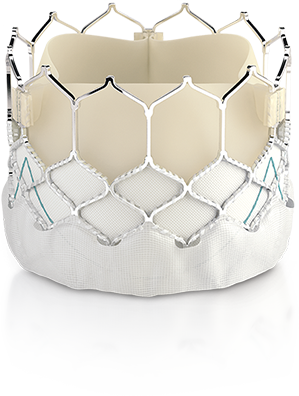
Edwards Lifesciences Corporation (NYSE: EW), the global leader in patient-focused innovations for structural heart disease and critical care monitoring, today announced that it has received CE Mark to expand use of the Edwards SAPIEN 3 transcatheter heart valve for the treatment of patients diagnosed with aortic stenosis who are at low risk for open-heart surgery. The Edwards SAPIEN 3 valve is the first transcatheter aortic valve implantation (TAVI) system to have this indication in Europe.
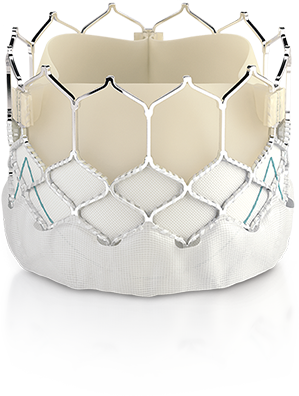
“Now, all European patients diagnosed with aortic stenosis can be considered for TAVI with the SAPIEN 3 valve based on factors such as anatomical considerations or other individual needs rather than risk scores,” said Prof. Helge Möllmann, Director, Clinic for Internal Medicine (Cardiology) at St. Johannes Hospital, Dortmund, Germany. “This is particularly important for patients at low risk for surgery, whose only serious health issue may be aortic stenosis and who want to return to their lives more safely and quickly. Previously, their only treatment option was open-heart surgery, and this approval will expand access to the proven SAPIEN 3 valve.”
This indication expansion in Europe follows on the publication earlier this year of data from the pivotal PARTNER 3 trial, an independently evaluated, randomized clinical trial comparing outcomes between TAVI and open-heart surgery in patients with a low surgical risk. TAVI with the SAPIEN 3 system achieved superiority, with a 46 percent reduction in the event rate for the primary endpoint of the trial, which was a composite of all-cause mortality, all stroke and rehospitalization at one year. The data were published in the New England Journal of Medicine.
An additional study examining quality of life in the PARTNER 3 patients, which was published online in the Journal of the American College of Cardiology, demonstrated significant early and sustained advantages for low-risk patients treated with the SAPIEN 3 valve. When the treatment strategies of TAVI and surgery were compared for low-risk patients, the TAVI patients improved more rapidly than surgery patients. This study showed, for the first time, patients treated with the SAPIEN 3 valve experienced a better quality of life even one year after the procedure.
The SAPIEN TAVI valves are the most widely studied transcatheter valves, with more than 30,000 patients treated in clinical trials and registries in over 65 countries around the world. Since the first commercial approval of the SAPIEN transcatheter valve in Europe in 2007, the SAPIEN family of valves have treated hundreds of thousands of patients worldwide. The more advanced SAPIEN 3 TAVI system, first approved in Europe in January 2014 for the treatment of high-risk patients and then expanded to intermediate-risk patients, builds on Edwards’ decades of experience in the development of tissue heart valves, and the proven benefits of the Edwards SAPIEN valves. While prior experience demonstrates that it takes some time for clinical practice to evolve, this new low-risk indication for the SAPIEN 3 valve in Europe should facilitate changes in clinical practice guidelines and reimbursement, to improve future access for patients.
See Full Press Release: Edwards SAPIEN 3 TAVI Receives Expanded Approval In Europe | Edwards Lifesciences
Written by: Edwards Lifesciences

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
