Medical device and MedTech insights, news, tips and more
EvoEndo Announces US FDA 510(k) Clearance for their Single-Use, Unsedated Transnasal Endoscopy System
February 17, 2022
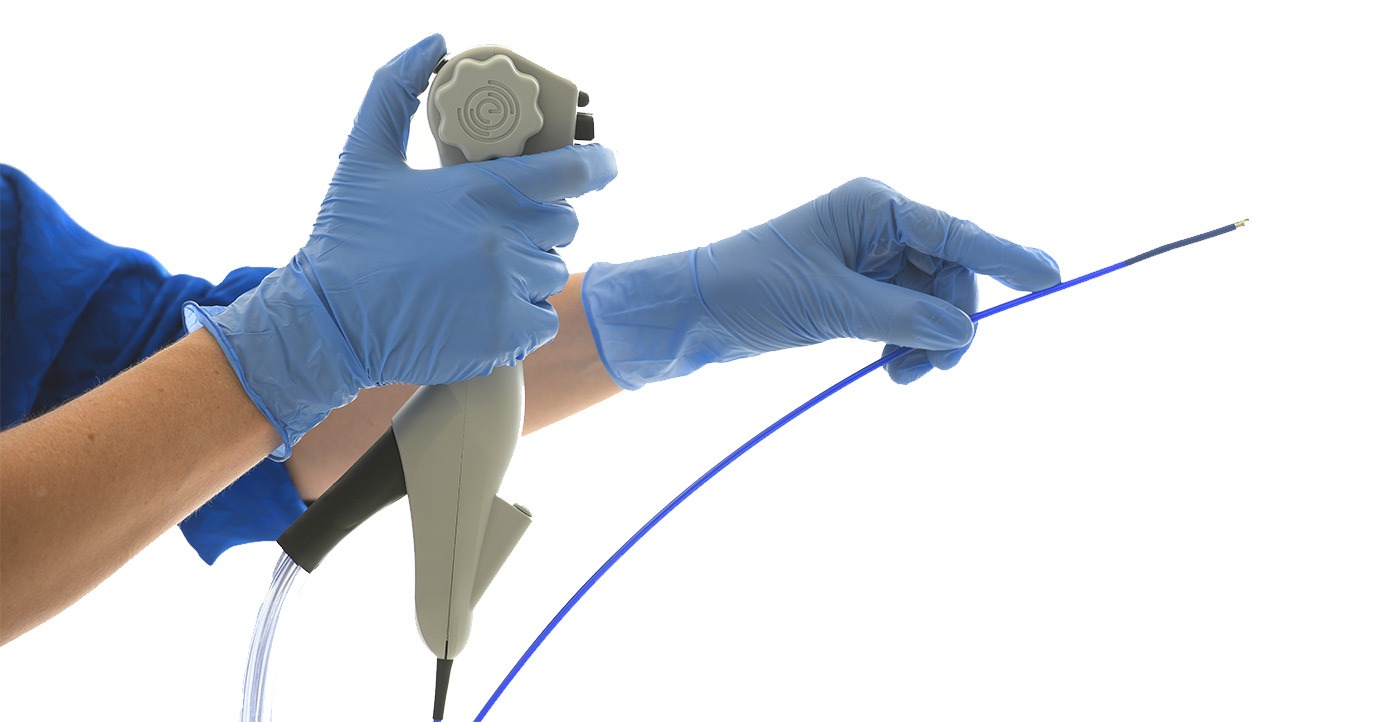
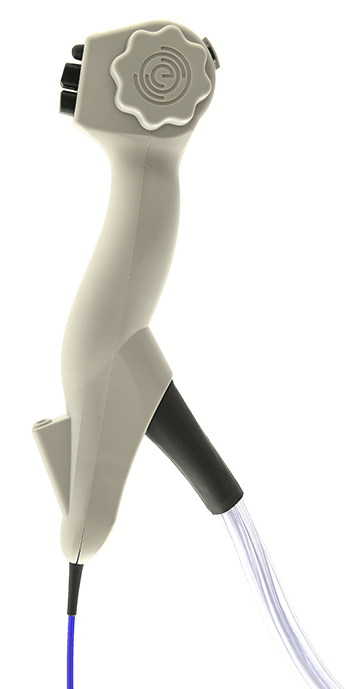
EvoEndo®, Inc. (“EvoEndo”), a medical device company developing systems for Unsedated Transnasal Endoscopy (TNE), has announced the receipt of 510(k) clearance from the U.S. Food and Drug Administration (FDA) to begin marketing and sale of the EvoEndo® Single-Use Endoscopy System. The clearance follows EvoEndo’s distribution agreement with Micro-Tech Endoscopy USA, Inc. (“Micro-Tech”), which will begin a phased distribution of the EvoEndo System into hospitals and ASCs in the United States.
EvoEndo was founded in 2017 by Dr. Joel Friedlander, a Pediatric Gastroenterologist at Children’s Hospital of Colorado, and is led by Chief Executive Officer Dr. Heather Underwood, an experienced medical device and technology entrepreneur, and alumna of the Stanford University Biodesign Program. While traditional endoscopy requires patients to undergo general anesthesia or sedation, the EvoEndo System combines sterile, single-use, flexible endoscopes, a portable video controller, and a take-home “comfort kit” containing virtual reality (VR) goggles for patient entertainment and distraction during the procedure to allow for unsedated transnasal endoscopy. The EvoEndo System ultimately enables safer and more cost-effective upper endoscopic procedures for patients, doctors, and hospitals. The FDA clearance is the latest milestone for EvoEndo, who also announced the completion of a $10.1M equity financing round last June.
Heather Underwood, Chief Executive Officer at EvoEndo, commented, “Receiving FDA 510(k) clearance for the EvoEndo System will allow us to execute on our mission of enabling a safer, faster, and more affordable alternative to sedated endoscopy for both pediatric and adult patients. This is an exceptional accomplishment for our team and validates our ongoing commitment to transform best practices in endoscopy and support the broader adoption of unsedated procedures throughout the U.S.”
“With today’s announcement, we are one step closer towards making unsedated endoscopies the standard of care within the medical community,” said Joel Friedlander, Chief Medical Officer and Co-Founder of EvoEndo. “We are thrilled to receive this clearance and proud to be on the forefront of a new and innovative system to help diagnose and treat pediatric and adult patients.”
“The EvoEndo® Model LE Single-Use Gastroscope addresses critical clinical needs in current pediatric and adult endoscopy practice and is a prime example of the innovative medical technology we strive to provide to our network,” stated Micro-Tech USA President Chris Li. “A combination of the smaller scope size, larger biopsy channel, coupled with a sterile single-use device can help save valuable procedure time and cost. We look forward to further growing our partnership with EvoEndo and to the successful completion of initial clinical cases.”
The EvoEndo System is only intended for use by medical professionals. Physicians and other medical providers interested in learning more about EvoEndo’s TNE system or to schedule demonstrations and training can contact the company here.
About EvoEndo®
EvoEndo®, Inc. is a medical device company developing systems that enable unsedated endoscopic procedures through a combination of sterile single-use, flexible endoscopes and VR-based patient distraction. EvoEndo’s technology allows pediatric patients and adults alike to receive routine endoscopies in a clinic setting without the use of general anesthesia or sedation, while reducing complexity, cost, and patient/provider apprehension. To learn more, please visit: https://evoendo.com/.
See Full Press Release at the Source: EvoEndo Announces US FDA 510(k) Clearance for Single-Use Unsedated Transnasal Endoscopy (TNE) System
Press Release by: EvoEndo®, Inc.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.