Medical device and MedTech insights, news, tips and more
FDA Approves Diffusion-Weighted Images for Elekta Unity, Expanding Options for Assessment During Therapy
December 16, 2019
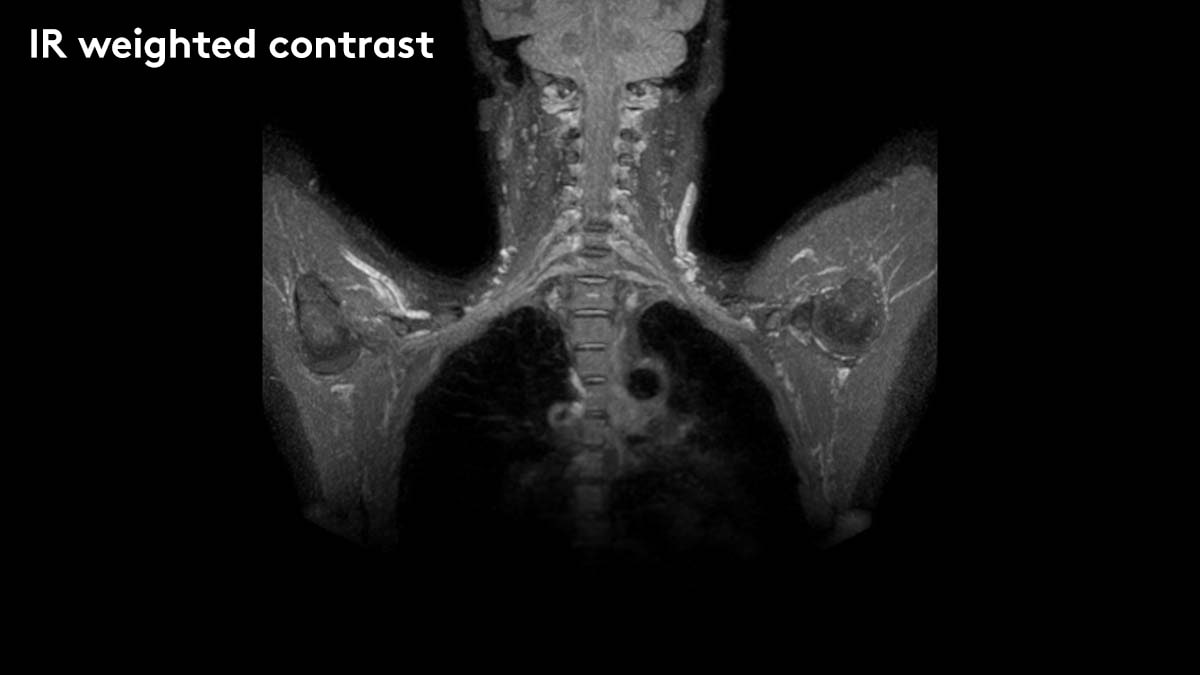
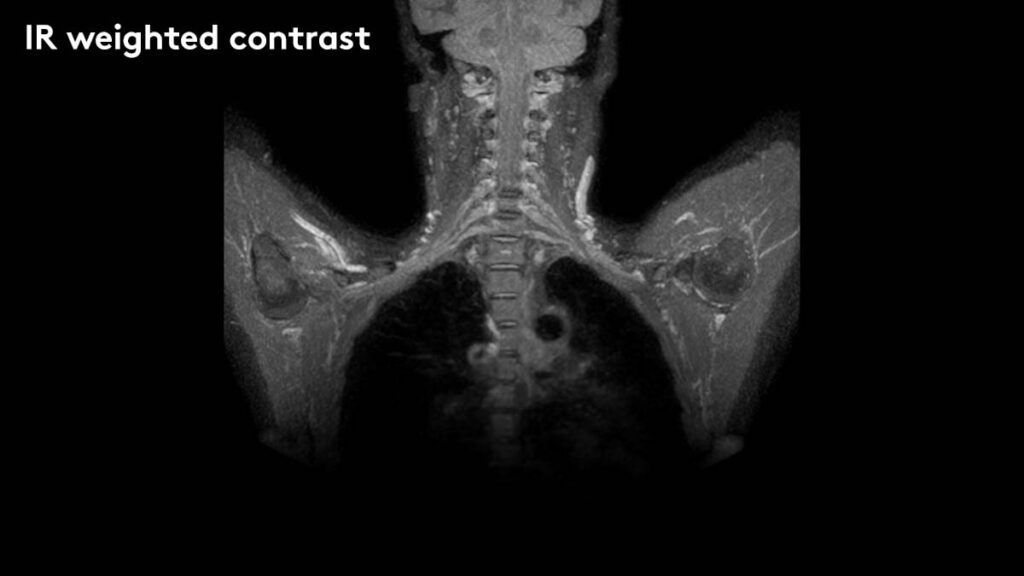
Elekta today announced that it has received 510(k) premarket notification from the U.S. Food and Drug Administration for the use of diffusion-weighted MR images (DWI) obtained with Elekta Unity to be interpreted by a trained physician. This expands the clinical utility of Elekta Unity to include biologic assessment of tumor response during therapy, allowing treatment adaptation based not just on gross anatomic changes but also on early biologic changes at the cellular level. Elekta Unity, the world’s first high-field MR-Linac, received initial 510(k) clearance in December 2018 and CE mark in June 2018.
DWI creates a map of the diffusion of water molecules at the cellular level and can be processed to generate the apparent diffusion coefficient, or ADC. A growing body of evidence shows that ADC changes within a tumor can provide important insights into an individual’s tumor response that would previously have been unavailable during radiation treatment. These insights can support further personalization of the radiation therapy regimen by allowing more tailored dose adaptation. The acquisition of DWI is critically dependent on the high quality of the 1.5 Tesla MR scanner integrated into Elekta Unity and can occur while the patient is undergoing their treatment with little or no overhead.
“One of the goals for Elekta Unity was to develop an MR-guided radiation therapy system that not only treats patients with unparalleled anatomic personalization but could also incorporate the individuals’ response to their treatment.,” said Richard Hausmann, President and CEO, Elekta. “This new functionality has excited early adopters of Unity. It allows us to assess biologic changes within the tumor, which may occur earlier than anatomic changes. This will improve clinicians’ ability to deliver the right dose to the right part of the tumor based on this new biological marker.
As a result of this 510(k) clearance, the Indication of Use Statement for Elekta Unity has been updated to include the following language: When interpreted by a trained physician magnetic resonance images acquired before, during, and after the radiotherapy treatment yield information that can be useful in diagnosis and may assist therapy planning, patient positioning and treatment delivery related to radiation oncology.
See Full Press Release: FDA Approves Diffusion-Weighted Images for Elekta Unity, Expanding Options for Assessment During Therapy
Written by: Elekta

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.