Medical device and MedTech insights, news, tips and more
FDA Approves Seno Medical’s Breast Lesion Diagnostic Technology
January 20, 2021
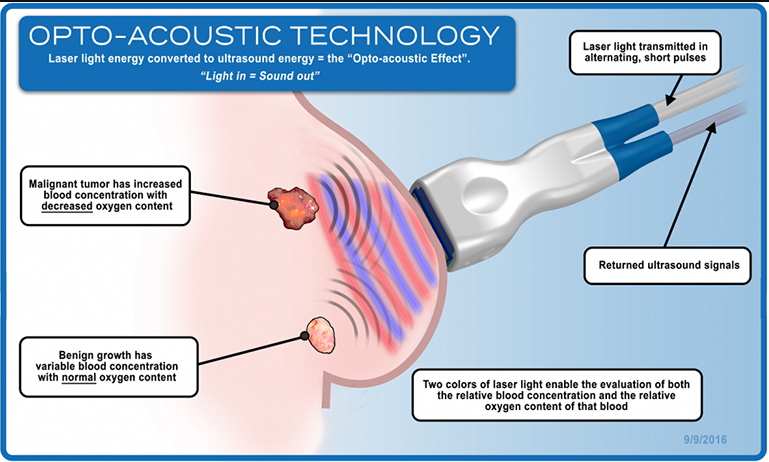
The Center for Devices and Radiological Health (CDRH) of the US Food & Drug Administration (FDA) has granted Texas-based Seno Medical Instruments, Inc. (Seno) premarket approval (PMA) for its groundbreaking diagnostic breast cancer imaging technology that helps physicians better differentiate between benign and malignant breast lesions. The company’s Imagio Breast Imaging System uses non-invasive opto-acoustic ultrasound (OA/US) technology to provide information on suspicious breast lesions in real time, helping providers characterize and differentiate masses that may – or may not – require more invasive diagnostic evaluation.
Breast biopsy procedures caused by false-positive diagnostic assessments in the United States cost the healthcare system more than $2 billion per year. Seno’s Imagio technology could mitigate that by giving providers additional real-time information regarding suspicious breast masses and increased confidence to make a better decision regarding the need for diagnostic breast biopsies.
The company’s OA/US technology combines laser optics and grayscale ultrasound to provide fused functional and anatomical breast imaging. The opto-acoustic images provide a unique blood map in and around breast masses, while the ultrasound provides a traditional anatomical image. Through the appearance or absence of two hallmark indicators of cancer – angiogenesis and deoxygenation – Seno Medical has shown that the Imagio OA/US Breast Imaging system will be a more effective tool to help radiologists confirm or rule out malignancy compared to traditional diagnostic imaging modalities – without exposing patients to potentially harmful ionizing radiation (x-rays) or contrast agents. In addition to the novel imaging provided by the Imagio system, Seno includes an AI physician decision support tool (the SenoGram) to aid in interpreting the new images that, along with training and certification, help radiologists make the transition from ultrasound alone to OA/US Imaging.
The system is indicated for use by trained and qualified healthcare providers to evaluate palpable and non-palpable breast abnormalities in adult patients who are referred for diagnostic imaging breast work-up following clinical presentation or other imaging examinations such as screening mammography.
Seno’s Chief Executive Officer Tom Umbel commented, “We are thrilled to have reached this milestone and are looking forward to moving our technology platform forward in the U.S. with this FDA approval. Our internal team and our faithful investigators and clinical trial sites have worked diligently to bring Imagio to market and improve care for patients and providers with the precise diagnostic capabilities and enhanced decision-making support that our novel OA/US imaging provides.”
Seno’s Chief Medical Officer, A. Thomas Stavros, MD, FACR, FSBI, believes that Imagio® is establishing a better standard for how diagnostic modalities should be judged. “Optimizing the diagnosis of breast masses requires a combination of very high sensitivity (≥98%) while simultaneously maximizing specificity and minimizing false positives and biopsies of benign masses. Other modalities have reported improvements in specificity, but these have often come at the expense of the desired high ≥98% sensitivity. The data from the PMA study shows that OA/US successfully achieved improved specificity at a fixed sensitivity of 98%, the part of the ROC curve where clinical decisions about whether or not to biopsy a mass are actually made.”
Seno Medical Instruments, Inc. is a San Antonio, Texas-based medical imaging company committed to the development and commercialization of a new modality in cancer diagnosis: opto-acoustic imaging. Seno Medical’s Imagio® Breast Imaging System fuses opto-acoustic technology with ultrasound (OA/US) to generate real-time functional and anatomical images of the breast. To learn more about Seno Medical’s OA/US imaging technology and applications, visit www.SenoMedical.com.
See Full Press Release at the Source: FDA Approves Seno Medical’s Ground-Breaking Breast Cancer Diagnostic Technology – Seno Medical
Press Release by: Seno Medical
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.