Medical device and MedTech insights, news, tips and more
FDA Clears Brainsway TMS System to Treat OCD
August 20, 2018
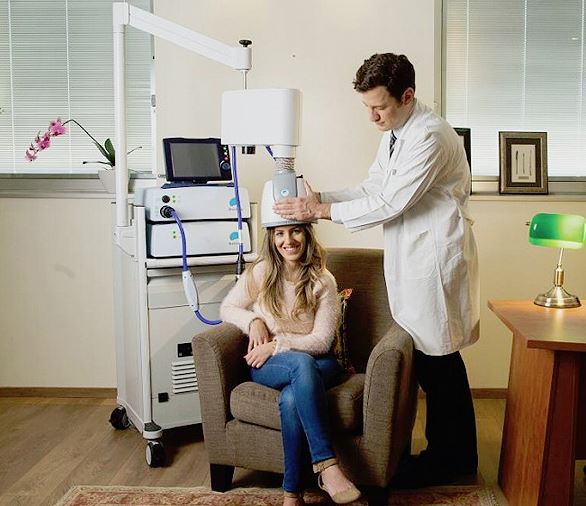
 On Friday, the FDA said it granted de novo approval for Brainsway‘s deep transcranial magnetic stimulation system, now indicated for treating obsessive compulsive disorder.
On Friday, the FDA said it granted de novo approval for Brainsway‘s deep transcranial magnetic stimulation system, now indicated for treating obsessive compulsive disorder.
The TMS system uses magnetic fields to simulate nerves in the brain, and has been shown to reduce the severity of OCD in patients.
A 100-patient randomized, multi-center study of the device indicated that patients who received TMS treatment alongside medical management had a higher response rate, at 38%, than those treated with medical management and a sham device, at 11%.
The most frequent adverse reaction was headache, which was reported by 37.5% of patients who received treatment with the Brainsway device and 35.3% of patients who received sham treatment. No serious adverse reactions were reported and other adverse reactions, including application site pain or discomfort, jaw pain, facial pain, muscle pain, spasm or twitching and neck pain were reported as mild or moderate and resolved shortly after treatment, according to the FDA.
Read More at the Source: Brainsway wins FDA nod for OCD-treating TMS system – MassDevice
By: Fink Densford
 A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
A Speciality Recruiting Firm Exclusively Servicing The Medical Device Industry
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.
