Medical device and MedTech insights, news, tips and more
FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions
January 29, 2020
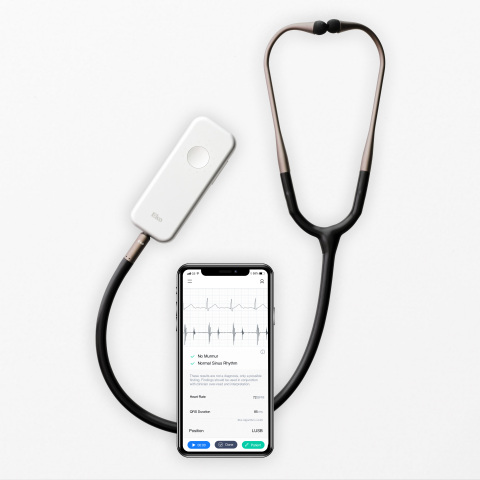
Eko, a digital health company applying artificial intelligence (AI) in the fight against heart disease, announced today that the U.S. Food and Drug Administration has cleared a suite of algorithms that, when combined with Eko’s digital stethoscopes, will enable healthcare providers in the U.S. to more accurately screen for heart conditions during routine physical exams. If left undiagnosed, these heart conditions can lead to stroke and heart failure.
“Our vision since day one has been to build seamless technology that helps providers more accurately detect heart disease, the leading killer in the world, by putting the ears of a cardiologist in any clinician’s stethoscope,” says Connor Landgraf, Eko’s Co-Founder and CEO. “Eko’s new ability to alert a provider to the presence of a heart murmur or atrial fibrillation during the standard physical exam brings that vision to life.”
Key Points:
- Patients with valvular heart disease and AFib often go undiagnosed during the physical exam. Murmurs indicative of valvular or structural heart disease can be extremely difficult for clinicians to hear and AFib may not be detected during the physical exam. AFib is an irregular and often rapid heartbeat that is often asymptomatic and can lead to blood clots, strokes, heart failure and other heart-related complications.
- Eko’s AI is able to identify heart murmurs, a leading symptom of valvular heart disease, with 87% sensitivity and 87% specificity. In comparison, a recent study revealed that using traditional stethoscopes, primary care physicians had a sensitivity of 43% and a specificity of 69% for detecting significant valvular heart disease, which affects over 5 million Americans.
- The AI is able to detect AFib with 99% sensitivity and 97% specificity when analyzing the 1-lead ECG tracing from the Eko DUO stethoscope. The integration of ECG into the stethoscope enables providers to quickly screen patients for the serious arrhythmia during a standard physical exam.
- The Algorithm also reports heart rate and QRS duration and identifies tachycardia and bradycardia, abnormally fast and slow heart rates, which can be indicative of heart disease or other health conditions such as thyroid disease.
“Two centuries after its invention, the stethoscope is still the front line tool to detect cardiovascular disease,” says Dr. Patrick McCarthy, Executive Director of the Bluhm Cardiovascular Institute at Northwestern Medicine and member of Eko’s Scientific Advisory Board. “Eko’s development of artificial intelligence algorithms to help clinicians better interpret sounds, identify arrhythmias and detect heart murmurs during a physical exam is going to make a huge difference in our ability to care for patients.”
Eko’s AFib and Murmur screening algorithms are the first in a suite of cardiac screening algorithms Eko plans to combine with its digital stethoscope devices to assist providers in the detection of cardiovascular conditions. In December 2019, Eko announced the FDA had granted the company breakthrough status for a novel ECG-based algorithm that, if FDA-cleared, could provide an easily accessible screening test for heart failure. Since the release of its first-generation Eko CORE device in 2015, Eko’s technology has been adopted by clinicians at more than 4,000 hospitals and clinics in the U.S. and Europe.
See Full Press Release: FDA Clears Eko’s AFib and Heart Murmur Detection Algorithms, Making It the First AI-Powered Stethoscope to Screen for Serious Heart Conditions | Business Wire
Written by: Eko Health

Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.