Medical device and MedTech insights, news, tips and more
FDA clears “first ever” 3D printed spine implant to treat of multiple injuries
January 19, 2018
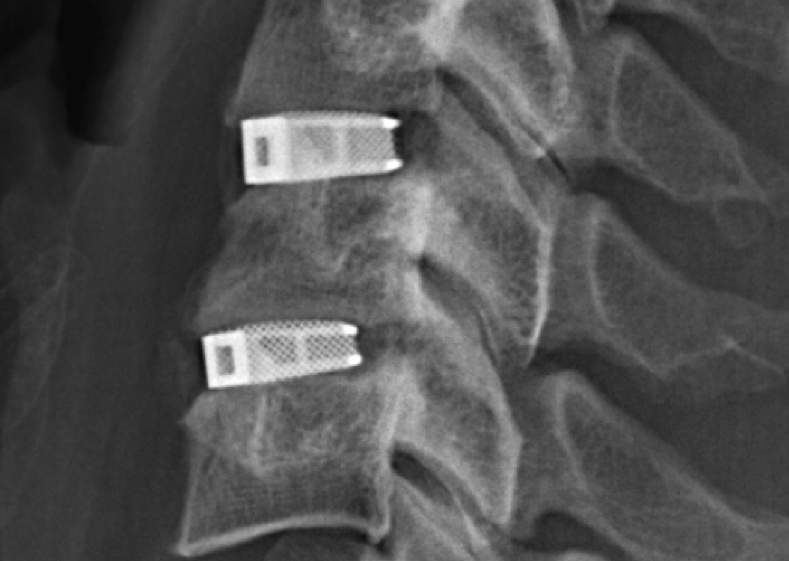
Metal additive manufacturing has hit on a clear niche within the healthcare industry for producing small and complex medical grade implants at scale. Many 3D printing companies working within medicine have a portfolio of devices for the purpose, typically including a sample of 3D printed cages used to support the spine.
However, in what is tipped to be a first in the U.S., the Food and Drug Administration (FDA) has cleared a 3D printed cervical device that can be used in a number of injuries/defects from the middle to the top of the spine.
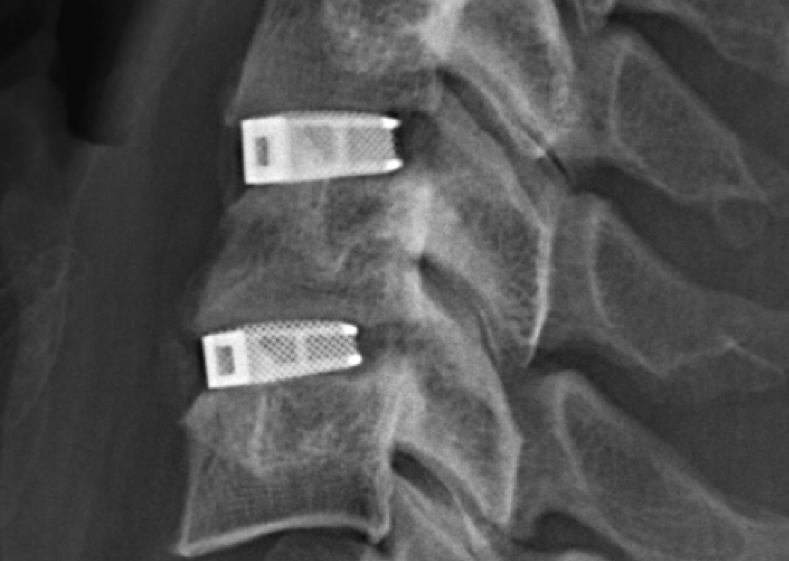
Made by Cellular Titanium technology
The multilevel cervical cage was made by Emerging Implant Technologies GmbH (EIT) using its trademark Cellular Titanium® technology.
Cellular Titanium devices are 3D printed in a process of selective laser melting (SLM). By precisely programming the laser’s movements, EIT is able to make implants with a core that mimics the cancellous (“spongy”) and cortical (hard) structure of bone.
When implanted in the body, biomimetic lattice-like structure ecourages tissues to grow through the center and graft broken pieces of bone back together.
7 levels of treatment
The remarkable quality of EIT’s cervial implant is that it is a 3D printed device that can be applied across 7 levels of damage to the spine.
In reference to the international chart for spinal cord injuries, EIT’s cervical implant can be applied to levels C2 to T/Th1 relating to the mid and upper segments of the spine. At the upper level (C2) the implant can be used to treat injuries to the auditory nerves, sinuses, eyes and tongue. The lower levels encompass the shoulders, neck, elbows, arms and fingers.
Read More at the Source: FDA clears “first ever” 3D printed spine implant to treat of multiple injuries – 3D Printing Industry
