Medical device and MedTech insights, news, tips and more
FDA Clears Siemens’ Cios Flow Mobile C-arm System
January 18, 2021
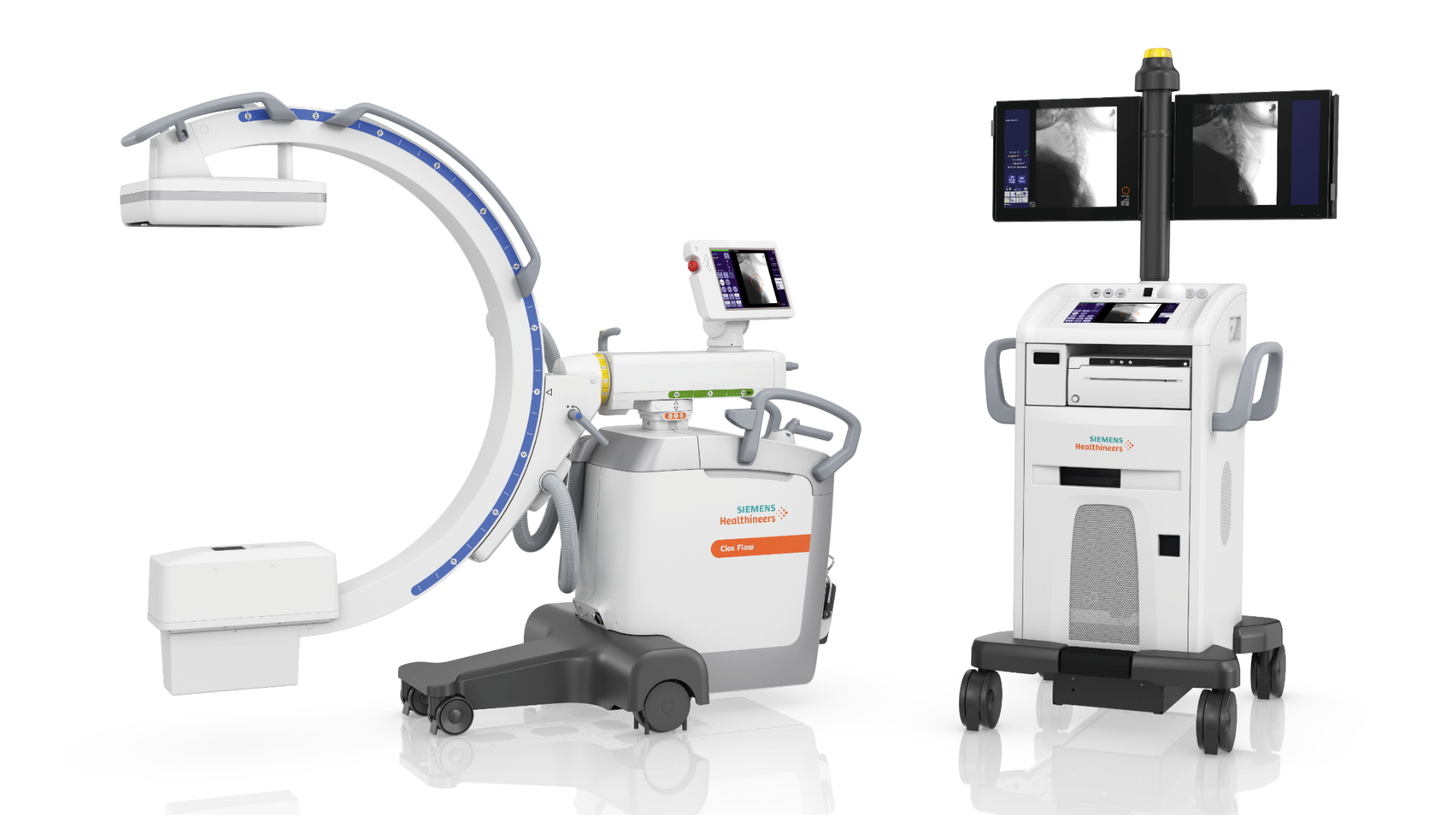
Siemens Healthineers has announced the Food and Drug Administration (FDA) clearance of the Cios Flow, a mobile C-arm designed for use by multiple disciplines in the operating room (OR) – including orthopedics, trauma surgery, spinal surgery, vascular surgery, and pain therapy – to increase the ease and efficiency of everyday imaging workflows for surgical interventions. This multidisciplinary functionality helps to ensure high-capacity system utilization, making the Cios Flow cost-effective. Additionally, its features enable easier, more efficient operation to improve patient care in the OR.
The Cios Flow’s low weight, maneuverability, and intuitive touch-gesture interface simplify operation. When the user taps a challenging anatomical area on the preview image of the Touch User Interface, the SpotAdapt function automatically optimizes relevant imaging and post-processing parameters such as brightness and contrast.
Additionally, the Cios Flow offers Windows 10 security functions to help minimize cyberattacks targeting the system or patient data, as well as Access & User Management features to prevent unauthorized access and restrict sensitive data. Applications can be whitelisted to ensure that the user accesses only safe software from trusted sources. Patient data can be encrypted using BitLocker, and system changes can be tracked.
“With the Cios Flow, Siemens Healthineers proudly offers customers a mobile C-arm system with broad multidisciplinary functionality as well as a robust level of cybersecurity to help enable secure, efficient operations in the OR,” said Lara Barghout, Senior Vice President of Advanced Therapies at Siemens Healthineers North America.
See Full Press Release at the Source: FDA Clears Cios Flow Mobile C-arm System From Siemens Healthineers
Press Release by: Siemens Healthineers
 Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.
Legacy MedSearch has more than 30 years of combined experience recruiting in the medical device industry. We pride ourselves on our professionalism and ability to communicate quickly and honestly with all parties in the hiring process. Our clients include both blue-chip companies and innovative startups within the MedTech space. Over the past 10 years, we have built one of the strongest networks of device professionals ranging from sales, marketing, research & , quality & regulatory, project management, field service, and clinical affairs.We offer a variety of different solutions for hiring managers depending on the scope and scale of each individual search. We craft a personalized solution for each client and position with a focus on attracting the best possible talent in the shortest possible time frame.
Are you hiring?
Contact us to discuss partnering with Legacy MedSearch on your position.

